Lumbar Disc Arthroplasty: History and Analysis
Alexandra C. Echevarria1*, Robert E. Carrier1, Anas M. Abbas1, Bongseok Jung1, Tim Reed2, Rohit B. Verma2
1Donald and Barbara Zucker School of Medicine at Hofstra/Northwell, Northwell Health, Hempstead, NY, USA
2Department of Orthopaedic Surgery, Northwell Health - North Shore University Hospital, Manhasset, NY, USA
Abstract
Degenerative disc disease describes a ubiquitous condition involving the natural deterioration of an intervertebral disc. Conservative methods such as physical therapy and anti-inflammatories are recommended as first-line therapies for noninvasive management. However, when these interventions fail to reduce pain, surgical intervention is indicated. While laminectomies, discectomies, and spinal fusion procedures have been considered the standard treatment throughout the 20th century, the development of artificial discs has introduced an alternative surgical intervention in the form of total disc replacement. Initially, the novel devices garnered significant attention, thus leading to a rise in the rates of disc replacement procedures performed. However, several years after FDA approval of the first device, the prevalence of procedures steadily decreased. Several factors may have contributed to the downward trend, including the growing financial burden of hospitalization, stringent inclusion criteria indicating the procedure, and lack of provider familiarity and comfort with the procedure. Although the expected prevalence of disc arthroplasty remains unrealized, there is significant potential for an expanded role in the contemporary treatment of degenerative disc disease. This review illustrates the timeline and course of lumbar disc arthroplasty by describing its development, followed by its introduction in Europe and eventual arrival in the United States. The initial growth in popularity due to promising results is explained along with the surgery’s swift decline primarily due to lack of sufficient evidence promoting replacement, poor insurance coverage, lack of clear indications and complications. This review encapsulates all components and describes future directions and clinical value of lumbar disc arthroplasty.
Introduction: History and Development of Lumbar Disc Replacement Devices
In 1960, Alf Nachemson was the first to measure intradiscal pressure for both preserved and moderately degenerated discs in cadaveric models. He later used this information to map mechanical load distribution on the human spine in vivo and pioneered many studies on the relationship between disc degeneration and musculoskeletal back pain1. In 1966, Ulf Fernström was the first to implant two stainless steel spheres in 191 lumbar and 13 cervical disc spaces of 125 patients. Having been inspired by the success of joint arthroplasty in knees and hips, Fernström’s aim was to construct an implant with a mobile center of rotation2. While initial clinical outcomes were comparable to fusion procedures in terms of functional outcomes, significant complications associated with subsidence and extrusion necessitating revision surgeries limited further research and application of the modality2.
The modern prosthetic discs used today originated with the development of the SB Charité intervertebral disk prosthesis by Dr Karin Buettner-Janz and Dr. Kurt Schellnack in 1982. The design featured two metal end-plates and an interposed, sliding polyethylene core. The construct was intended to maintain the motion-sparing elements of its predecessors while conferring improved stability at the interface between the prosthesis and native vertebral endplates3. Initial ex vivo biomechanical testing offered promising results. When embedded in polypropylene, the prostheses were found to function adequately under a high static load and under a lighter dynamic load for a long time. However, subsequent testing in cadaveric models revealed challenges with subsidence of the constructs limited by a need for particularly large end-plate components3. Refinement of this design over the next six years culminated in the SB Charité III, which became commercially available in France in 1989. The initial evaluation of the device was promising, with a cohort of 93 patients reporting significant pain reduction accompanied by improvements in lumbar mobility and ambulatory capacity in comparison to the non-intervention cohort. Unfortunately, the small sample size and limited follow-up period for the study were indicative of the need for higher-powered investigation with long-term follow up to better assess efficacy and feasibility of disc replacement procedures4.
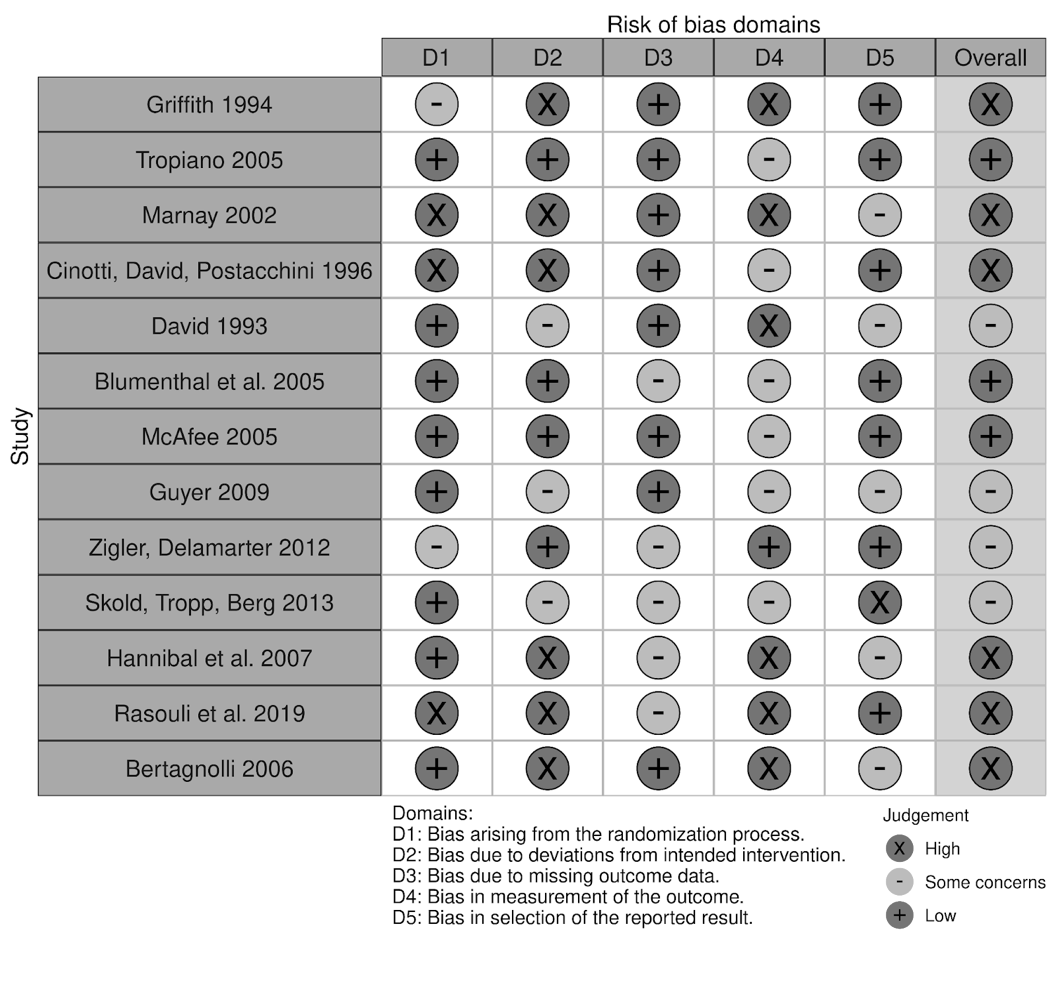
Despite the lack of studies assessing the long-term patient outcomes associated with disc replacements, positive results in preliminary studies contributed to the growing excitement around disc arthroplasty as a non-inferior alternative to fusion procedures. What followed was a rise in interest throughout the 1990s as multiple new disc prostheses were developed with variations in design, structure, materials, and biomechanical properties5. Following the emergence and first implantation of the ProDisc I model in 64 patients in 1990, the ProDisc II was developed and stood alongside the SB Charité III model as one of the flagship models of the 1990s5-7. Publications related to the various device models during this period became increasingly prevalent. In fact, European data proved particularly invaluable to facilitating the adoption of the technology by demonstrating low complication rates associated with the use of the devices10,11. Despite the smaller sample sizes and observational character of these studies, promising outcomes of lumbar total disc replacements (L-TDRs) continued to contribute to growing interest, especially in the United States. It was this interest, and perhaps the overall positive 7-11 year follow up results with ProDisc, that led to the unusually rapid FDA approval of the ProDisc-L12 (Table 1). A risk of bias assessment was conducted for this study along with other referenced original studies and is summarized in Table 2. The authors agree much stronger, longer-term studies were needed at the time. If more efficient studies were conducted earlier, the devices may have gained more traction over a longer period.
Prospective, randomized, multicenter FDA Investigational Device Exemption (IDE) studies for the Charité disc began in 2000 and reported overall favorable results for L-TDR patients in comparison to Anterior lumbar interbody fusion (ALIF), with shorter recovery times and greater patient satisfaction at 24 months follow up13. Radiographic outcomes were positive, with preservation of preoperative range of motion on flexion/extension views, restoration of disc space height, and less subsidence than ALIF with Bagby and Kuslich (BAK) cages14. Additionally, five-year follow up of these patients reported no significant radiographic changes in the L-TDR group between two and five years follow up, and clinical outcomes between L-TDR and ALIF with BAK group were not statistically different15 (Table 1). Charité was later approved for use in 2004.
In 2001, a decade after the first ProDisc I implantation had occurred in Europe, the Prodisc-L began its FDA-IDE trial and was then approved in 2006. Multiple other lumbar arthroplasty devices were developed during this period. While all the novel devices became available outside of the United States, few received FDA approval and were discontinued or withdrawn from trial. Despite the shortcomings of their competitors, the Charité and Prodisc models continued to generate enthusiasm for the clinical and financial potential of disc arthroplasty. Singh et al. predicted that half of all spinal arthrodesis procedures would be replaced by L-TDR and that the disc arthroplasty market in the United States would be worth over $2 billion by 200816. This burgeoning interest in the field was reflected by an exponential increase in related publications in the United States during the 2000s. From 1999-2008, 102 surgical outcome studies, 63 clinical reviews, and 46 biomaterial and biomechanical reports were published17. Despite the initial interest and research related to disc arthroplasty in the European sphere, the majority of the most-cited articles on disc arthroplasty originated from the United States18. In 2011, Johnson & Johnson acquired rights to the Prodisc-L and it’s competitor, the Charité, was discontinued just one year later23.
Table 1: Review of original studies referenced comparing device implants used on human patients to identify postoperative outcomes
|
Study |
Study Type |
Study Size |
Average age of patients |
Indication for surgery |
Treatment Groups |
Results |
Complications |
|
Griffith et al. (1994)3 |
Retrospective Cohort |
142 |
Males - 43.7 ± 7.9 years of age.
Females - 42.3 ± 6.7 years of age. |
DDD1(65.2%) |
LINK SB Charite III v. Charite I and II |
Resolution of preoperative back pain in 19.8% of patients (P < 0.05). Improved neurologic weakness, decreased pain on straight leg raise, increased overall walking ability, and increased lumbar mobility (P < 0.01) |
Mechanical failure of model III implant prosthesis in 1 patient. Inappropriate prosthetic size in 6.5.% of patients. Reoperation rate of 10% in patients treated with Models I and II and 3% in those treated with Model III. |
|
Marnay (2002)6 |
Prospective Cohort |
64 |
- |
Chronic low back pain |
Pro-disc implant |
64.7% improvement in back pain scores, 73% improvement in VAS2 scores, and 65% long term satisfaction. |
7.8% of patients experienced ongoing pain necessitating posterior spinal fusion. |
|
Tropiano et al. (2005)7 |
Prospective Cohort |
55 |
46 years of age |
DDD1 and severe lumbar pain |
Pro-disc implant |
Significant postoperative improvements in low back and leg pain (p < 0.0001). |
Increased radicular pain postoperatively due to nerve root traction in 9% of patients. |
|
Cinotti, David, Postacchini (1996)10 |
Retrospective Cohort |
46 |
36 years of age |
DDD1 and painful discography at the affected level |
Charite SB III disc |
Satisfactory clinical results in 69% of single level cases and in 40% of double level cases (P = 0.004). |
Malposition of the prosthesis in 6.5% of patients and subsidence of the implant in 8.6%. |
|
David (1993)9 |
Prospective Cohort |
22 |
37 years of age |
Lumbar and/or radicular chronic pain |
Charite SB III disc |
65% of patients reported excellent or good post-operative outcomes. |
Severe sciatica in 1 patient after the implant was inserted into a narrow disc space. |
|
Blumenthal et al. (2005)12 |
Prospective RCT |
304 |
39.6 years of age |
Single level DDD1 |
TDR3 with Charite SB III v. ALIF6 with BAK threaded fusion cages |
Both groups had significant improvement in ODI4 and VAS scores at all time points (P =0.05). At 12 months, patients in the Charite group had greater rates of satisfaction (P = 0.001). Significantly lower rate of pain medication use at 24 months in the investigational group (P =0.0428) |
No significant difference in complications between the two groups (P = 0.4484) |
|
McAfee (2005)13 |
Prospective RCT |
205 |
39.6 years of age |
Single level DDD1 |
TDR3 with Charite SB III v. ALIF5 with BAK cages packed with autograft |
At 24 months, the preoperative ROM6 increased by 13.6% in the investigational group. Significantly less subsidence in the investigational group (P < 0.05) |
None |
|
Guyer (2009)15 |
Prospective RCT |
277 |
39.6 years of age |
Single level DDD1 |
Charite Artificial disc v. ALIF5 with BAK cages. |
Overall success rate was 57.8% in the Charite group and 51.2% in the BAK group. At 5 year follow-up, the Charite group had greater overall spine ROM6. |
None |
|
Zigler, Delamarter (2012)28 |
Prospective RCT |
236 |
Fusion group - 40.4 ± 7.6 years of age TDR3 group - 38.7 ± 8.0 years of age |
Single level DDD1 |
TDR3 with Pro-DiscL v. Fusion |
Mean operative time was significantly shorter in the TDR3 group (P < 0.00001). Both groups maintained improvement in ODI4 at 5 years (P <0.0001) and in the SF-36 PCS7 at 2 and 5 years (P < 0.0001). At 2 years, the TDR3 group had improved neurological responses (P = 0.0341). |
None |
|
Skold, Tropp, Berg (2013)31 |
Prospective RCT |
152 |
TDR group - 40.2 ± 8.1 years of age, Fusion group 38.5 ± 7.8 years of age |
DDD1 |
TDR3 (Charite, Prodisc, or Maverick) v. Fusion |
At 5-years, 38% of the TDR3 group reported total pain relief compared to 15% of the fusion group. Back pain and ODI4 improvement at 5 years was greater in the TDR group (P = 0.006). |
No significant difference in complications between the two groups. |
|
Hannibal et al. (2007)32 |
Prospective cohort |
59 |
39 years |
1-2 level lumbar DDD1 |
1 level Prodisc replacement v. 2 level ProDisc replacement |
1-level procedures had a 47% decrease in VAS2 scores compared to 37% decrease in 2-level cases at 2 years. No significant difference in SF-367 scores between 1 and 2-level cases (P=0.37) |
A tear in the iliac vein identified in a 1-level disc replacement while 2 patients experienced postoperative radiculopathy. In 2-level surgeries, 2 patients experienced foot drop. |
|
Rasouli et al. (2019)34 |
Prospective Cohort |
159 |
41 years of age |
DDD1 at 2 or more contiguous levels |
2, 3, and 4 level ProDisc-L disc replacements |
All groups had a statistically significant improvement in ODI4 and VAS2 scores. No difference in ROM6 improvement postoperatively. |
In the 2-level group, 1 patient experienced continued back pain, and another had the prosthesis removed due to dislocation. One patient in the 3-level group underwent a posterior fusion for continued back pain. |
|
Bertagnolli (2006)37 |
Prospective cohort |
22 |
63 years of age |
Disabling discogenic low back pain with L5-S1 DDD1 |
ProDisc-L |
Statistically significant improvement in VAS2 and ODI4 scores at baseline and 3 months (P< 0.00001). Motion increased from 3 degrees to 12 degrees postoperatively (P<0.004). |
There were 2 cases of implant subsidence and 2 cases of foot drop. |
1(DDD): Degenerative Disc Disease, 2(VAS): Visual Analog Scale, 3(TDR): Total Disc Replacement, 4(ODI): Oswestry Disability Index, 5(ALIF): Anterior Lumbar Interbody Fusion, 6(ROM): Range of Motion, 7(SF-36 PCS): Short Form-36 Physical Component Summary.
Table 2: Risk of bias assessment of the referenced studies identifying patient outcomes following implant placement
Decline in Incidence of Lumbar Disc Replacement
Although the emergence of these devices and the corresponding expansion of published literature in North America during the 1990s and 2000s indicated significant interest in total disc arthroplasty as an alternative to fusion procedures, retrospective data collected during this timeframe suggests this interest may not have been borne out in practice. Analysis of the Nationwide Inpatient Sample (NIS), the largest inpatient healthcare database in the United States, revealed that surgical treatment for degenerative disc disease (DDD) increased 2.4-fold in the United States. However, the rates of lumbar arthrodesis that coincided with this growth from 2000 to 2008 were not matched by disc arthroplasty19. In fact, while there was a 10% increase in lumbar fusion procedures, there was a 28% decrease in lumbar arthroplasty procedures between 2005 and 200820. The diversion of patients away from arthroplasty and toward arthrodesis procedures may be, in part, explained by growing interest in other surgical techniques that were being developed in the early 2000’s. Techniques such as percutaneous posterior lumbar spinal fusion, anterior interbody fusion, and direct lateral interbody fusions were seeing a rise in incidence at this time20. The authors postulated that the majority of the decline was due in part to high power studies demonstrating clear clinical benefit of lumbar arthroplasty versus arthrodesis.
The number of L-TDRs continued to decline over the next decade, with Mills et al. observing a steep decline from 2009 through 2017, by which time only 600 cases of L-TDR were estimated to have been performed annually21. Overall, there was an 82% decrease in L-TDR from 2005-2017 which represented a sharp contrast to the exponential increase in lumbar fusions conducted during the same time21. Part of this discrepancy may have been rooted in the demonstration of equivalent outcomes and complication rates between lumbar fusion and L-TDR in this timeframe21. In 2008, Harrop et al. published a systematic review which established lumbar fusion procedures as superior in terms of decreasing segmental disease rates22. There was also a geographic variation in the number of the L-TDRs performed that should be noted. In the years 2005-2009 and 2010-2017, while there was a significant decrease in L-TDRs performed in the Northeastern, Midwestern, and Southern United States, the rates in the Western region of the country remained relatively constant. It is the authors’ opinion that this may be explained by a higher number of high-volume spine centers in the region.
A retrospective study performed by Saifi et al. evaluated the NIS database to research the trends in LDA procedures performed between 2005 and 2013. The study found that during this time, while the total annual number of revision procedures needed decreased by 30.4%, the incidence of primary L-TDRs decreased by 86%, which may in and of itself, explain the reduction in required reoperation23. The revision rates for L-TDR were higher than those observed after lumbar fusion, which provided further support for fusion procedures. It is important to note, however, that the national revision burden of L-TDR in the U.S. falls within the acceptable range, especially when compared to other procedures which have higher revision rates such as hip and knee replacements. This contradictory evidence requires further exploration to determine true cost benefits for lumbar arthroplasty versus arthrodesis.
Additionally, the higher revision burden for L-TDR is offset by the lower average hospital cost of primary TDR and revision procedures compared to fusion procedures24,25.
A more recent analysis compared the outcomes of patients who underwent single and double-level TDR with those who had lumbar fusion procedures performed between the years 2010 and 2019. The study showed that patients who had undergone single-level TDR procedures had a significantly shorter length of hospital stay, lower rates of discharge to healthcare facilities, and lower total healthcare cost when compared with matched lumbar fusion patients. Single-level TDR patients also had significantly lower rates of operative complications including the need for blood transfusion and neurologic complications. In comparing two-level L-TDR and lumbar fusion patients, there were no significant differences in length of hospital stay, rates of discharge to other facilities, or rates of complication. However, the total cost of care for L-TDR patients was significantly higher ($53,270 vs. $44,721, P=0.005), representing a reversal of the previously observed cost-benefit of L-TDR detailed in preceding studies25,27. The study further followed the frequency of L-TDR procedures performed and saw a similar plateau between the years 2010 and 2013 but noted a slight increase in 2019, at which time it accounted for 0.74% of operations for lumbar DDD. At that time, the proportion of two-level procedures increased from 15.7% in 2010 to 45.5% in 201927. Although rates of L-TDR procedures performed have seen fluctuations since the early 2000’s, the operation is shown to have several benefits in patient outcomes. However, the realization of these benefits has been in part hindered by financial burden at which they may come.
There are multiple reasons that may explain the slow acceptance of L-TDR during this period. Hart et al. conducted a nationwide survey that found that a large proportion of spine surgeons had not adopted the procedure despite having the appropriate training and experience, citing limited indications, concerns regarding long-term complications, technical challenges in revising a failed prosthesis, and poor coverage by insurance providers24. This final point was one of the most frequently endorsed deterrents, as 78.6% of insurers included in that assessment provided no coverage for L-TDR. This lag in coverage may in part be attributed to the fact that the North American Spine Society (NASS) coverage policy recommendations for L-TDR for single level disease were not released until 2014, a full 10 years after FDA approval of the Charité device. The recommendations for L-TDR were advanced single level disease of the lumbar spine characterized by moderate to severe degeneration of the disc with Modic changes as compared to other normal or mildly degenerative levels. The recommendations also called for patients to have symptoms refractory to non-operative management with physical therapy for a minimum of 6 months24. It makes sense that the lack of recommendations would be a notable deterrent for actually performing the procedure.
Indications and Contraindications for Lumbar Total Disc Replacement
Current indications for L-TDR include symptomatic DDD refractory to nonoperative interventions for a minimum of 6 months, preoperative Visual Analog Scale (VAS) back pain scores of at least 40-50/100, preoperative Oswestry Disability Index scores > 40%, a measured T score > - 1.0, and localization of the pathology to the L4-L5 and L5-S1 disc levels28. Contraindications include joint compromise secondary to other degenerative anatomic abnormalities such as scoliosis, stenosis, spondylolisthesis, and severe facet arthritis, as well as patient age > 60 serving as a relative contraindication29. The narrow inclusion criteria yielded from these studies may in part represent a limiting factor in the lack of widespread adoption of the procedure at present. However, post FDA-IDE trial research suggests there may not need to be such stringent inclusion criteria when indicating patients for L-TDR. For example, Bertagnoli et al. proposed that patients older than 60 years of age may still represent appropriate surgical candidates provided overall bone health is sufficient and there is no evidence of the prohibitive anatomic abnormalities described above30. Additionally, the development of prophylactic vertebral body augmentation has been found to be associated with lower risk of subsidence or failure of TDR in patients with low T scores, opening the door for TDR as a viable treatment option for patients with osteoporosis/osteopenia17,30.
Insurance Coverage
As the first FDA-approved disc prosthesis, the Charité artificial disc perhaps bore a disproportionately large burden of responsibility in the adoption of L-TDR in the United States. Following its FDA approval in 2004, the Centers for Medicare and Medicaid Services ruled that there was insufficient evidence to conclude that lumbar artificial disc replacement with the Charité artificial disc was reasonable and necessary treatment warranting coverage. The lack of coverage through Medicare and Medicaid services in the early adoption stage after initial release not only reduced patient access to L-TDR as a treatment modality, but may have secondarily diminished confidence among surgeons, patients, and private insurance providers. The authors believe that lack of coverage may have stymied the momentum that L-TDR had in the United States despite the lack of narrow inclusion criteria. Between 2005 and 2013, private insurance comprised 52% of payer types for L-TDR surgeries, although most private insurance companies did not provide any coverage or reimbursement for the procedure26. Due to the lack of insurance coverage, the decreased popularity of L-TDR may be expected with the demand for the procedure undermined by the lack of financial support. The lag in insurance coverage can be partly explained by the fact that the FDA only granted approval for single-level procedures despite there being several studies revealing double-level L-TDRs to be noninferior to fusion procedures. This is because the original FDA-IDE trials tested only single-level arthroplasty and arthrodesis surgeries.
With these FDA studies serving as the foundation upon which coverage and reimbursement largely became based, the ability to offer further develop disc arthroplasty procedures was limited17. Although several studies supporting more inclusive criteria have since been published, there remains a lag between updated guidelines and approved coverage by insurance companies. The percentage of insurance providers covering TDR has seen only a gradual increase with 13%, 25%, and 65% of insurance providers covering a single-level lumbar arthroplasty in 2012, 2015, and 2020, respectively23.
Complications of L-TDR
Although L-TDR procedures have been found to be efficacious in terms of pain alleviation and functional improvement, the limited index of longitudinal studies revealing potential long-term complications of the procedure remains a deterrent to providers and, in turn, their patients. L-TDR complications are divided into technique, or device-related complications. Due to the technically challenging nature of a T-LDR procedure, technique complications such as mispositioning of the device are of particular concern. In the case of the ProDisc II, 29.3% of patients with facet degeneration following L-TDR were found to have mispositioned device placement in a 5-10 year follow-up study17. Additional technique-related complications include facet joint distraction, fractures, and device dislocations. The device does not have the inherent mobility of a healthy vertebral joint, and stress placed on the construct by natural body dynamics may lead to the aforementioned complications. Device-specific complications were particularly prevalent in earlier models, for example, the ProDisc-L was associated with split fractures of the vertebral body. Since the Pro-Disc-L is a dual-keel model, the minimal total height of the 2 keels facing each is 13 mm, and when the sagittal height of a vertebral body falls to less than 26mm, over half of the vertical dimension in the midsagittal plane is occupied by keel. These 2 keels driven into smaller opposing chisel cuts create opposing wedge that can split the vertebral body23,46. Beyond the complications associated with the primary procedure, complications associated with a revision procedure must also be considered especially with the installation of and potential need for removal of hardware. In L-TDR cases where revision procedures are indicated, the treatment options are to either replace the implant entirely or to proceed with lumbar fusion. The L-TDR revision procedures are associated with higher incidence of vascular injuries and other complications due to the challenging nature of the procedure. Because of this, 52.7% of surgeons surveyed communicated having reservations about the challenges of the revision surgery after L-TDR23. Overall revision rate rates in one study were reported to be 6-14% and the study noted risks of the procedure such as surgical site infections, major vessel bleeding, and pseudoarthrosis39. The revision burden for TDR was found to increase from 11.6% in 2005 to 24.3% in 2013 which is a critical value to weigh, especially when considering patient selection for the primary procedure26. The complications related to both primary and revision procedures have played and will continue to play an integral role in the adoption of L-TDR.
Rising Interest in Lumbar Fusion
In 2004 and 2005, randomized control trials with a 2-year follow-up revealed TDR to be a noninferior alternative to lumbar fusion. These initial studies may have been a driving force for the initial peak in popularity of the L-TDR procedure. However, in 2008, Harrop et al. conducted a systematic review to evaluate the incidence of adjacent segment disease following arthrodesis or TDR. When considering the rates of symptomatic adjacent segment disease and disc degeneration, the study made the lowest tier recommendation for L-TDR over arthrodesis25. In general, studies have been unable to consistently offer clear evidence supporting the superiority of L-TDR in comparison to fusion which has hindered the acceptance of L-TDR. Specifically, short-term benefit of the L-TDR has rarely been borne out over the long-term. An analysis of the readmission rates of patients who underwent L-TDR and fusion procedures showed no difference between the two procedures. While L-TDR was associated with lower rates of revision surgeries at 90-day and 1-year follow-up, there were no significant differences seen in rates of subsequent surgeries at 3- and 5-year follow-up between the two groups40. In 2016, a meta-analysis conducted by Bai et al. found there to be a significant difference in the VAS scores between L-TDR and lumbar fusion in favor of L-TDR at a 1-year follow-up, yet there was no significant difference by 24 months. With regard to ODI scores, there was greater improvement among patients in the L-TDR group initially but after 24 months, there was, again, no significant difference between the two groups40.
Between the years 1998 and 2008, there was a 2.7-fold increase in primary lumbar fusion surgeries coinciding with contemporary advances made in lumbar fusion techniques, occurring around the same time as L-TDR procedures were growing in popularity. Reisener et al. predicted consistent increases in the rates of lumbar fusion procedures performed due to the reduced complication rates, more minimally invasive approach, and level of precision during instrumentation40. Lumbar fusion procedures saw a two-fold increase in the number of procedures supported by Medicare, rising from 21% in 1998 to 40% by 201443. In the late 2000s, several advances were made in the realm of lumbar fusion surgery which occurred around the same time as the decrease and eventual plateau seen in L-TDR procedures performed. These included novel approaches, such as the direct lateral interbody fusion, and techniques such a percutaneous pedicle screw placement44. Upon analysis of the time frame where lumbar fusion surgeries saw an increase in rate and where L-TDR procedures began to decrease, the literature suggests there is a correlation between the two. The novel techniques involved in lumbar fusion procedures, lack of long-term differences in VAS and ODI scores between L-TDR and lumbar fusion procedures, as well as more optimal insurance coverage for fusions likely all contributed to the decreased trends in L-TDR procedures performed.
Future Directions and Conclusion
L-TDR has progressively evolved over the past four decades and will continue to do so. Most recent designs provide a substantial improvement over previous versions with safer complication profiles and more effective motion preservation to prevent adjacent segmental disc disease. The critical element of a lumbar disc is the preservation of normal, anatomic motion. In adhering to native joint kinematics, the risk of adjacent segment disease and subsequent surgical revision should theoretically be mitigated23,31. One recent study conducted by Zot et al. in 2023 aimed to analyze the ROM of modern prostheses. They developed patient specific FE models, which were derived from CT scans of human lumbar specimens, to analyze mechanical effects linked to arthroplasty in lumbar levels. Their results found that elastic prostheses most efficiently replicated native discs and restored normal ROM in patients with degenerative disease but found ball-and-socket prostheses reduce loads at the lower adjacent level and increase ROM at the index level in patients without degenerative disease45.
With new technologies and biologics, comes more efficient lumbar constructs. For example, utilizing preoperative planning and three-dimensional printing to generate patient specific implants to better conform endplates with native anatomy could help improve device functionality and longevity while decreasing subsidence issues23. Furthermore, the growing interest in a familiarity with L-TDR procedures may be expected to continue given the FDA’s approval of two-level L-TDR for the ProDisc-L in 2020 after a long-term study demonstrated no significant differences between one- and two-level arthroplasty as well as the Society for the Advancement of Spine Surgery’s statement of support for L-TDR as an alternative to lumbar fusion in 202127,28,32. Although more consistent data is still required for one and two level L-TDRs, there have also been recent attempts at evaluating three or more contiguous vertebral levels. In evaluating and comparing two- to four-level L-TDR in their cohort of patients, Rasouli et al. found significant reductions in both the ODI and VAS scores34.
Another interesting development in the world of arthroplasty is the concept of performing hybrid arthroplasty alongside fusions. This procedure entails either placing an artificial lumbar disc adjacent to a previous fusion or performing the procedures simultaneously34,35. Early trials have demonstrated promising results35,26. In a more recent study in 2022 by Young et al, authors compared patients undergoing single level L-TDR, multi-level L-TDR, or hybrid construct procedures between 1998-2012. Patient-reported outcome measures in all groups showed statistically and clinically significant improvements in pain and function well above the corresponding minimum clinically important difference (MCID) and exceeding literature thresholds for substantial clinical benefit (SCB). They also were able to establish the hybrid construct as superior to multi-level LDA in terms of Roland Morris Disability Questionnaire scores at 6 months follow-up40.
Overall, the reasons for the declining popularity of L-TDR in the 2000s and early 2010s are likely due to inconsistent clinical results, difficulties with insurance authorization, concerning complications, advancements made in alternative procedures, and narrow inclusion criteria without recent studies attempting to expand the indications for this procedure24. This calls the real clinical value of lumbar disc arthroplasty into question. There have been many studies displaying superior results to arthrodesis, but overall, the evidence points to non-inferior outcomes. Since the lumbar arthrodesis procedure is more familiar to surgeons, there are reservations that accompany the transition to performing a more contemporary surgical approach. Overall, the guiding clinical value is the health of the patient, and until there is a clear benefit of LDA, arthrodesis will remain the standard. Multiple long-term studies of L-TDRs have begun to alleviate some of the concerns showing improved clinical benefits and safety profiles at five years and beyond and have demonstrated significant differences in reoperation rates and patients satisfaction favoring arthroplasty to arthrodesis16,23,30. However, there continues to be a need for recent, long-term, high-power studies to evaluate new emerging designs and technologies that support the use of L-TDR. With renewed interest in the field, research-bolstered validation of favorable longitudinal outcomes and increasing financial support from insurance providers may point to a future of lumbar disc replacement brighter than its previous trends would suggest.
Author Contributions
The authors confirm contribution to the paper as follows: Conception of study and design: Robert Carrier; Data collection and literature search: Alexandra Echevarria, Anas Abbas, Bongseok Jung; Data interpretation: Alexandra Echevarria, Robert Carrier; Draft Manuscript preparation: Alexandra Echevarria, Robert Carrier; Critical revision of the article: Rohit Verma, Tim Reed; Final approval of the version to be published: Robert Carrier, Alexandra Echevarria, Anas Abbas, Bongseok Jung, Tim Reed, Rohit Verma
Disclosures
- No funding was received for this study.
- The authors have no competing interests to declare.
- The study was not registered.
- A protocol was not prepared.
Conflicts of Interest
The authors of this review do not have any financial, professional, or personal conflicts to report.
References
- Rydevik B, Hansson T, Szpalski M, et al. Alf Nachemson, MD, PhD, 1931-2006: an exceptional pioneer in spine care. Eur Spine J. 2007; 16(3): 303-305. doi:10.1007/s00586-007-0330-1
- Büttner-Janz K, Schellnack K, Zippel H. Biomechanics of the SB Charité lumbar intervertebral disc endoprosthesis. Int Orthop. 1989; 13(3): 173-176. doi:10.1007/BF00268042
- Griffith SL, Shelokov AP, Büttner-Janz K, et al. A multicenter retrospective study of the clinical results of the LINK SB Charité intervertebral prosthesis. The initial European experience. Spine (Phila Pa 1976). 1994; 19(16): 1842-1849. doi:10.1097/00007632-199408150-00009
- Büttner-Janz K, Schellnack K. A Modulartyp SB Charité porckorong endoprothesis elve és alkalmazásának elsö eredményei [Principle and initial results with the Charité Modular type SB cartilage disk endoprosthesis]. Magy Traumatol Orthop Helyreallito Seb. 1988; 31(2): 136-140.
- Lemaire JP, Skalli W, Lavaste F, et al. Intervertebral disc prosthesis. Results and prospects for the year 2000. Clin Orthop Relat Res. 1997; (337): 64-76. doi:10.1097/00003086-199704000-00009
- Marnay T. Lumbar disc replacement: 7 to 11-year results with Prodisc. Spine J. 2002; 2: 94S.
- Tropiano P, Huang RC, Girardi FP, et al. Lumbar total disc replacement. Seven to eleven-year follow-up. J Bone Joint Surg Am. 2005; 87(3): 490-496. doi:10.2106/JBJS.C.01345
- Szpalski M, Gunzburg R, Mayer M. Spine arthroplasty: a historical review. Eur Spine J. 2002; 11 Suppl 2(Suppl 2): S65-S84. doi:10.1007/s00586-002-0474-y
- David T. Lumbar disc prosthesis. Surgical technique, indications and clinical results in 22 patients with a minimum of 12 months follow-up. Eur Spine J. 1993; 1(4): 254-259. doi:10.1007/BF00298370
- Cinotti G, David T, Postacchini F. Results of disc prosthesis after a minimum follow-up period of 2 years. Spine (Phila Pa 1976). 1996; 21(8): 995-1000. doi:10.1097/00007632-199604150-00015
- Delamarter RB, Bae HW, Pradhan BB. Clinical results of ProDisc-II lumbar total disc replacement: report from the United States clinical trial. Orthop Clin North Am. 2005; 36(3): 301-313. doi:10.1016/j.ocl.2005.03.004
- Blumenthal S, McAfee PC, Guyer RD, et al. A prospective, randomized, multicenter Food and Drug Administration investigational device exemptions study of lumbar total disc replacement with the CHARITE artificial disc versus lumbar fusion: part I: evaluation of clinical outcomes [published correction appears in Spine. 2005 Oct 15; 30(20): 2356]. Spine (Phila Pa 1976). 2005; 30(14): 1565-E391. doi:10.1097/01.brs.0000170587.32676.0e
- McAfee PC, Cunningham B, Holsapple G, et al. A prospective, randomized, multicenter Food and Drug Administration investigational device exemption study of lumbar total disc replacement with the CHARITE artificial disc versus lumbar fusion: part II: evaluation of radiographic outcomes and correlation of surgical technique accuracy with clinical outcomes. Spine (Phila Pa 1976). 2005; 30(14): 1576-E390. doi:10.1097/01.brs.0000170561.25636.1c
- Guyer RD, McAfee PC, Banco RJ, et al. Prospective, randomized, multicenter Food and Drug Administration investigational device exemption study of lumbar total disc replacement with the CHARITE artificial disc versus lumbar fusion: five-year follow-up. Spine J. 2009; 9(5): 374-386. doi:10.1016/j.spinee.2008.08.007
- Singh K, Vaccaro AR, Albert TJ. Assessing the potential impact of total disc arthroplasty on surgeon practice patterns in North America. Spine J. 2004; 4(6 Suppl): 195S-201S. doi:10.1016/j.spinee.2004.07.009
- Beatty S. We Need to Talk about Lumbar Total Disc Replacement. Int J Spine Surg. 2018; 12(2): 201-240. Published 2018 Aug 3. doi:10.14444/5029
- Zhao T, Shen J, Zhang J, et al. Top 100 Cited Articles on Spinal Disc Arthroplasty Research. Spine (Phila Pa 1976). 2020; 45(21): 1530-1536. doi:10.1097/BRS.0000000000003608
- Yoshihara H, Yoneoka D. National trends in the surgical treatment for lumbar degenerative disc disease: United States, 2000 to 2009. Spine J. 2015; 15(2): 265-271. doi:10.1016/j.spinee.2014.09.026
- Awe OO, Maltenfort MG, Prasad S, et al. Impact of total disc arthroplasty on the surgical management of lumbar degenerative disc disease: Analysis of the Nationwide Inpatient Sample from 2000 to 2008. Surg Neurol Int. 2011; 2: 139. doi:10.4103/2152-7806.85980
- Hart RA, DePasse JM, Daniels AH. Failure to Launch: What the Rejection of Lumbar Total Disk Replacement Tells us About American Spine Surgery. Clin Spine Surg. 2017; 30(6): E759-E764. doi:10.1097/BSD.0000000000000415
- Harrop JS, Youssef JA, Maltenfort M, et al. Lumbar adjacent segment degeneration and disease after arthrodesis and total disc arthroplasty. Spine (Phila Pa 1976). 2008; 33(15): 1701-1707. doi:10.1097/BRS.0b013e31817bb956
- Saifi C, Cazzulino A, Park C, et al. National Trends for Primary and Revision Lumbar Disc Arthroplasty Throughout the United States. Global Spine J. 2018; 8(2): 172-177. doi:10.1177/2192568217726980
- Upfill-Brown A, Policht J, Sperry BP, et al. National trends in the utilization of lumbar disc replacement for lumbar degenerative disc disease over a 10-year period, 2010 to 2019. J Spine Surg. 2022; 8(3): 343-352. doi:10.21037/jss-22-4
- Kurtz SM, Lau E, Ianuzzi A, et al. National revision burden for lumbar total disc replacement in the United States: epidemiologic and economic perspectives. Spine (Phila Pa 1976). 2010; 35(6): 690-696. doi:10.1097/BRS.0b013e3181d0fabb
- Sandhu FA, Dowlati E, Garica R. Lumbar Arthroplasty: Past, Present, and Future. Neurosurgery. 2020; 86(2): 155-169. doi:10.1093/neuros/nyz439
- Mills ES, Shelby T, Bouz GJ, et al. A Decreasing National Trend in Lumbar Disc Arthroplasty. Global Spine J. 2023; 13(8): 2271-2277. doi:10.1177/21925682221079571
- Schroeder GD, Vaccaro AR, Divi SN, et al. 2021 Position Statement from the International Society for the Advancement of Spine Surgery on Cervical and Lumbar Disc Replacement. Int J Spine Surg. 2021; 15(1): 37-46. doi:10.14444/8004
- Zigler JE, Delamarter RB. Five-year results of the prospective, randomized, multicenter, Food and Drug Administration investigational device exemption study of the ProDisc-L total disc replacement versus circumferential arthrodesis for the treatment of single-level degenerative disc disease. J Neurosurg Spine. 2012; 17(6): 493-501. doi:10.3171/2012.9.SPINE11498
- Büttner-Janz K, Guyer RD, Ohnmeiss DD. Indications for lumbar total disc replacement: selecting the right patient with the right indication for the right total disc. Int J Spine Surg. 2014; 8: 12. doi:10.14444/1012
- Kitzen J, Vercoulen TFG, van Kuijk SMJ, et al. Long-term clinical outcome of two revision strategies for failed total disc replacements. Eur Spine J. 2020; 29(7): 1536-1543. doi:10.1007/s00586-019-06184-x
- Sköld C, Tropp H, Berg S. Five-year follow-up of total disc replacement compared to fusion: a randomized controlled trial. Eur Spine J. 2013; 22(10): 2288-2295. doi:10.1007/s00586-013-2926-y
- Hannibal M, Thomas DJ, Low J, et al. ProDisc-L total disc replacement: a comparison of 1-level versus 2-level arthroplasty patients with a minimum 2-year follow-up. Spine (Phila Pa 1976). 2007; 32(21): 2322-2326. doi:10.1097/BRS.0b013e3181557c06
- Fiani B, Nanney J M, Villait A, et al. Investigational Research: Timeline, Trials, and Future Directions of Spinal Disc Arthroplasty. Cureus. 2021; 13(7): e16739. doi:10.7759/cureus.16739
- Rasouli A, Cuellar JM, Kanim L, et al. Multiple-Level Lumbar Total Disk Replacement: A Prospective Clinical and Radiographic Analysis of Motion Preservation at 24-72 Months. Clin Spine Surg. 2019; 32(1): 38-42. doi:10.1097/BSD.0000000000000704
- Chen B, Akpolat YT, Williams P, et al. Survivorship and clinical outcomes after multi-level anterior lumbar reconstruction with stand-alone anterior lumbar interbody fusion or hybrid construct. J Clin Neurosci. 2016; 28: 7-11. doi:10.1016/j.jocn.2015.10.033
- Scott-Young M, McEntee L, Schram B, et al. Concurrent Use of Lumbar Total Disc Arthroplasty and Anterior Lumbar Interbody Fusion: The Lumbar Hybrid Procedure for the Treatment of Multilevel Symptomatic Degenerative Disc Disease: A Prospective Study. Spine (Phila Pa 1976). 2018; 43(2): E75-E81. doi:10.1097/BRS.0000000000002263
- Bertagnoli R, Yue JJ, Nanieva R, et al. Lumbar total disc arthroplasty in patients older than 60 years of age: a prospective study of the ProDisc prosthesis with 2-year minimum follow-up period. J Neurosurg Spine. 2006; 4(2): 85-90. doi:10.3171/spi.2006.4.2.85
- Yoder JH, Auerbach JD, Maurer PM, et al. Augmentation improves human cadaveric vertebral body compression mechanics for lumbar total disc replacement. Spine (Phila Pa 1976). 2010; 35(9): E325-E331. doi:10.1097/BRS.0b013e3181cf7055
- Scott-Young M, Lee SMS, Nielsen D, et al. Comparison of Mid- to Long-term Follow-up of Patient-reported Outcomes Measures After Single-level Lumbar Total Disc Arthroplasty, Multi-level Lumbar Total Disc Arthroplasty, and the Lumbar Hybrid Procedure for the Treatment of Degenerative Disc Disease. Spine (Phila Pa 1976). 2022; 47(5): 377-386. doi:10.1097/BRS.0000000000004253
- Reisener MJ, Pumberger M, Shue J, et al. Trends in lumbar spinal fusion-a literature review. J Spine Surg. 2020; 6(4): 752-761. doi:10.21037/jss-20-492
- Eliasberg CD, Kelly MP, Ajiboye RM, et al. Complications and Rates of Subsequent Lumbar Surgery Following Lumbar Total Disc Arthroplasty and Lumbar Fusion. Spine (Phila Pa 1976). 2016; 41(2): 173-181. doi:10.1097/BRS.0000000000001180
- Bai DY, Liang L, Zhang BB, et al. Total disc replacement versus fusion for lumbar degenerative diseases - a meta-analysis of randomized controlled trials. Medicine (Baltimore). 2019; 98(29): e16460. doi:10.1097/MD.0000000000016460
- Momin AA, Steinmetz MP. Evolution of Minimally Invasive Lumbar Spine Surgery. World Neurosurg. 2020; 140: 622-626. doi:10.1016/j.wneu.2020.05.071
- Sullivan HG, Bertagnoli R, Nigogosyan MA, et al. Prevention of vertebral body-splitting fractures after multilevel ProDisc-L implantation. Int J Spine Surg. 2012; 6: 93-102. doi:10.1016/j.ijsp.2011.12.004
- Zot F, Ben-Brahim E, Severyns M, et al. Study of mechanical effects of lumbar disc arthroplasty on facet joints at the index level/adjacent levels by using a validated finite element analysis. Front Bioeng Biotechnol. 2023; 11: 1287197. doi: 10.3389/fbioe.2023.1287197
