A Modified Study Design for Blinded Randomized Controlled Trial in Orthobiologic Therapy
Shaimaa Hassoun1, Jennifer Arthurs1, Shane Shapiro2*, Michael Heckman3
1Center for Regenerative Biotherapeutics, Mayo Clinic, Jacksonville, FL, USA
2Department of Orthopedic Surgery, Mayo Clinic, Jacksonville, FL, USA
3Division of Biomedical Statistics and Informatics, Mayo Clinic, Jacksonville, FL, USA
Abstract
Background: The utility of cellular based therapeutic agents in management of various ailments and conditions is promising, particularly in the field of orthopedics. However, an evidence-based medicine approach must be implemented to validate these novel cellular based therapies before they can be translated into routine clinical practice. Given pain relief is a primary goal of novel treatments for orthopedic disease, future orthobiologic clinical trials will need to overcome challenges such as the placebo effect or the placebo response and difficult participant recruitment. In this paper, we describe a clinical study that evaluates the safety and efficacy of autologous stromal vascular fraction (SVF) cells that adheres to a patient blinded, randomized and placebo-controlled study design while still offering the patient the opportunity to participate in the therapeutic intervention by using cell preservation techniques.
Methods: This pilot clinical trial studies the safety and feasibility of intra-articular transplantation of SVF cells in patients with knee osteoarthritis with secondary outcomes of improving pain relief. The RCT aims to recruit 30 patients. Liposuction is performed on each patient to isolate SVF cells. 15 subjects are randomized to receive SVF injection in the same surgical procedure and 15 subjects receive placebo injection directly into their affected knee joint. The placebo group’s SVF cells are then frozen and preserved. At the 6-month follow-up visit, those who received the placebo are unblinded and have the option to receive the SVF injection as a condition for participation in the trial. Feasibility of liposuction, SVF manufacturing, cell preservation/thaw techniques and adverse outcomes are all primarily tracked. Secondary outcomes include standardized patient reported outcomes score responses to the treatments.
Discussion: The design of this pilot study offers study subjects the opportunity to receive a novel therapeutic intervention even within a placebo arm and enables the investigators to blind subjects without performing an unnecessary liposuction or discarding the resulting cellular product which could encourage hesitant individuals to participate in the trial. This may aid in overcoming the challenges associated with recruiting participants for cell therapy trials concerned with being randomized to a control arm. The results of this trial will help to assess both the safety and feasibility of SVF injections to treat knee osteoarthritis as well as help plan larger phase controlled trials.
However, precautionary measures are necessary to ensure the safety and well-being of patients receiving cell-based therapy. Proper handling and storage of the cellular product must be considered and are demonstrated here.
Trial Registration
ClinicalTrials.gov Identifier: NCT03940950
Background
Osteoarthritis (OA) and chronic tendinopathies are leading causes of pain and disability worldwide1,2. The development of novel orthobiologic therapies has led to the hope of fulfilling gaps in treatment. Some first-generation therapies such as platelet rich plasma (PRP) have reasonable level 1 evidence to support therapeutic use in knee arthritis and some tendinopathies such as rotator cuff disease and lateral epicondylitis3,4. Bone marrow aspirate concentrate (BMAC), stromal vascular fraction, and microfragmented adipose tissue (MFAT) have demonstrated pain relief and improvement in quality of life in some clinical trials; however, others have failed to define such relief as superior to placebo or control injections and therefore require further study before they can enter routine use5-12. The randomized controlled trial is the gold standard for establishing therapeutic efficacy of a product or intervention which is often compared to placebo or standard of care13. However, when establishing the efficacy of a pain relieving therapeutic, the placebo effect or placebo response can make differentiating a therapeutic effect for novel pain relieving treatments difficult12,14-17. An additional challenge includes the recruitment of subjects for clinical trials when they know they might not receive the studied therapeutic intervention.
Future orthobiologic clinical trials will need to overcome these challenges if they are to validate these novel therapies and translate them into routine clinical practice18. In this paper, we describe and advocate for a clinical study design for cell therapies that adheres to the blinded randomized placebo-controlled trial while still offering the patient the opportunity to participate in the therapeutic intervention by using cell preservation techniques19.
Methods/Design
Study Design
This study is an ongoing pilot clinical trial studying the safety and feasibility of intra-articular transplantation of stromal vascular fraction (SVF) in patients with knee OA at Mayo Clinic’s Florida campus. Stromal vascular fraction is created from the enzymatic digestion of lipoaspiration adipose tissue with proposed therapeutic potential20,21 in clinical and pre-clinical investigations of osteoarthritis5,22,23. Our study design adheres to the guidance of Standard Protocol Items: Recommendations for Interventional Trials (SPIRIT) Statement (see supplement- SPIRIT Checklist)24,25. The study was approved by the Institutional Review Board at the Mayo Clinic and received Investigational New Drug authorization from the FDA. Patients seeking treatment for unilaterally painful, primary, femorotibial knee osteoarthritis are offered standard conservative medical therapy for their disease as part of routine orthopedic treatment. As an alternative, and if they have tried and failed conservative therapy, patients are informed of the investigational study protocol, and offered the option to enroll if they meet study criteria.
Pertinent participant inclusion criteria are Kellgren Lawrence Grade 2-3 osteoarthritis, male or female ages 18-75, previous 3 month or longer trial of one of the following conservative treatments: activity modification, weight loss, physical therapy, anti-inflammatory medications or injection therapy (e.g. cortisone, hyaluronic acid/viscosupplement), able to routinely walk without assistance, clinically stable target knee, completed general physical evaluation with primary care provider within 12 months of enrollment, full understanding of the requirements of the study and ability to give informed consent. Pertinent exclusion criteria include KL Grade 1 or 4, rheumatoid arthritis, diabetes or any clinically significant medical comorbidity, pregnant or nursing, or planning on becoming pregnant during the study period, congenital or acquired malformation of the target knee resulting in significant deformity, significant clinical malalignment requiring follow-up full length, standing X-rays, orthopedic hardware or implantable devices in the knee, surgery on the index knee within 1 year of study enrollment, injections of any into the index knee within 3 months prior to study enrollment, major mechanical symptom of the target knee, history of intra-articular infection in the index knee, history of falls requiring medical attention, or gait instability, abnormal hematology (complete blood count with differential), blood chemistry (Glucose, Calcium, Sodium, Potassium, Bicarbonate, Chloride, BUN, Creatinine, Anion Gap), urinalysis, aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase (ALP), and CRP, Body mass index (BMI) > 40 kg/m2, taking anticoagulant medications (e.g. warfarin, heparin) or clopidogrel (Plavix).
Upon obtaining informed consent of the participant, the trial enters the randomization phase. The Study Data Management System (SDMS) is used to computer-generate random numbers and assign the arm of study to a subject. Study participants are assigned randomly to either the placebo or SVF (biologic) treatment group. This unbiased process ensures the allocation lacks bias and predictability.
A total of 30 subjects will be enrolled and treated in this pilot study. The primary goal of this pilot study is to assess safety and feasibility of SVF, with statistical comparisons of outcomes between the two study groups considered as secondary and exploratory only. For this reason, the sample size of 30 patients is appropriate for such a pilot study, and was not chosen based on formal power analysis for the primary goal was not to identify statistically significant differences between the two treatment groups. However, it should nonetheless be mentioned that with a sample size of 15 patients in each of the two treatment groups, we will have 80% power at the 5% significance level to detect an effect size (Cohen’s d) of 1.1 using a two-sample t-test.
The study is conducted on an outpatient basis. All 30 patients enrolled undergo a liposuction procedure. The lipoaspirate is processed for SVF cell isolation. Each patient is blinded to the treatment they receive and randomized in a 1:1 fashion to either SVF or placebo intra-articular injection in the osteoarthritic knee performed under ultrasound guidance. Randomization is computer-based using the dynamic allocation method of Pocock and Simon, with stratification factors for Kellgren-Lawrence grade and prior knee surgery26.
Single-blinding among participants was also employed to reduce biases. In this arrangement, individuals are unaware of their specific treatment group, while investigators and healthcare providers possess knowledge of the group assignments. Participants in the placebo group are administered an intra-articular placebo injection that mimics the biologic treatment in appearance (and administration) but lacks the active therapeutic components, and the placebo control group’s cells from SVF are preserved in a sterile technique by Mayo Clinic Florida’s Human Cell Therapy Lab (HCTL). The 15 patients who are assigned to the SVF group receive SVF immediately after pre-determined quality controls demonstrate that the manufactured product meets pre-release criteria. The outcomes from these 15 patients treated with SVF will be compared to the 15 patients treated with a lactated ringer (LR) control. Additional (secondary) measures being collected include the Knee Injury and Osteoarthritis Outcome Score (KOOS), and a 100 mm Visual Analogue Scale (VAS) pain scores at baseline, 1 week, 6 weeks, 6 months, 12 months, and 2 years.
At the week 26 visit, subjects who received LR injections at baseline visit are unblinded and given the choice to receive their SVF injection as a therapeutic treatment. (Figure 1) In this manner, the trial can be a randomized and blinded controlled trial and still allow all patients to receive cell therapy. Control group subjects can only receive the study product at six months post-procedure if they are reassessed and continue to meet the study eligibility criteria prior to administration of the SVF. PROMs for the control group are not analyzed at 12 month and 24 month intervals because of the potential for the thawed SVF injection to confound the data and the resulting preserved SVF cells are not meant to serve as an independent intervention influencing patient outcomes.
Throughout the trial, participants are diligently monitored and assessed at predetermined intervals to gauge specific outcomes and detect any adverse events. Standardized methods and instruments are employed to collect data on efficacy, safety, and other pertinent parameters. The SVF will not be administered to subjects who were allocated initially to the control group if there is no preliminary evidence of bioactivity after the first few patients are treated or if safety issues have been identified.
Upon completion of the trial, the amassed data from both groups will be analyzed for differences in adverse events, cell characteristics and the secondary outcomes of pain and function.
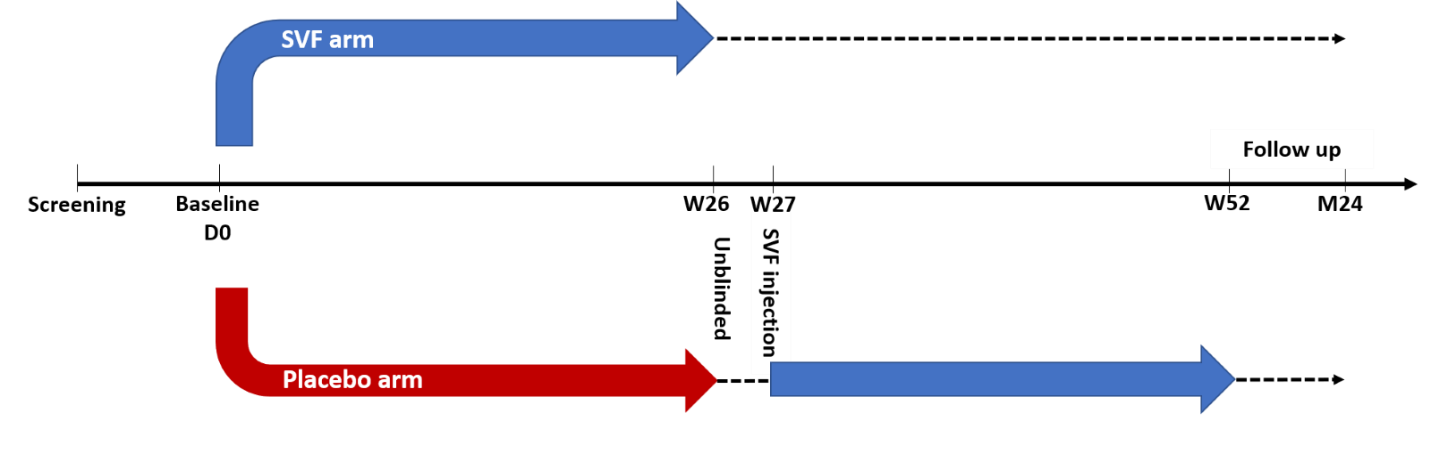
Figure 1: Study design timeline (D0: day 0, W26: week 26, W27: week 27, W52: week 52, M24: month 24) Week 27 – 24 month follow up for placebo arm is for safety only and PROMs are not compared to SVF arm.
Manufacturing
Lipoaspiration
Tumescent liposuction is used to collect up to 200 ml of adipose tissue from the subject's abdomen, flank, or buttocks under local anesthesia (1% lidocaine). This involves infusing a standard wetting solution through small incisions made with a #11 scalpel blade using a standard multi-hole infusion cannula. The wetting solution consists of 1 liter LR, 50 ml of 1% lidocaine, and 1 cubic centimeter of 1:1000 epinephrine, which is infused into both the deep and superficial fat compartments using a super-wet plus technique (2 volumes of wetting solution to 1 volume of proposed fat aspirate). After allowing 20 minutes for the vasoconstrictive effect of the epinephrine, the adipose tissue is aspirated.
Post aspiration, the excess lipoaspirate liquid portion (blood and tumescent solution) is removed by decantation. The lipoaspiration sites are sutured with an intradermal absorbable suture. The patient is prescribed an oral antibiotic for 2 days after the aspiration as part of standard care.
SVF cell isolation
A proprietary automated processor and closed loop system is used in this study for automated SVF cell isolation from human lipoaspirate. Adipose tissue is delivered to the processing chamber via input nozzle with a standard catheter tip syringe, minimizing potential contamination of the sample. The entire process is completely standardized and automated in a closed system. The KISO system (KEIA Vancouver, B.C.) employs a disposable tissue processing bag kit, Roche Liberase (MTD C/T, GMP Grade Item #06 297 790 001) enzyme and a standard bag of LR solution. The all-in-one 3 chamber tissue processing bag provides a contamination-free safe environment with consistency between multiple isolations. It contains chambers for (1) adipose tissue digestion, and separation of crude SVF from adipocytes; (2) waste collection; (3) further SVF purification by filtration, concentration, and collection.
Movement of fluids between the chambers is mediated by gravity eliminating the need for a pump and is controlled by single stopcock valve integrated in processing chamber. The resulting product is removed from the KISO device and transferred equally into two conical centrifuge tubes then centrifuged (1200 g, 10 min) to remove the fluid and recover SVF cell pellet. The isolated SVF cells are then re-suspended in an additional 10 ml LR and centrifuged again at 1200g for 5 minutes to remove any final cellular clumps and debris. The resulting SVF cells are then suspended in LR to total 10 ml for pre-release testing and injection. This sample of SVF in LR is the final product to be injected into the participant’s osteoarthritic knee joint. Total preparation time for the SVF is approximately 1 hour and cost, while dependent on several variables including surgical procedure, disposables, reagents and testing is approximately five thousand dollars per treatment.
Preservation
The resulting SVF product from the control group is centrifuged at 500 x g for 5 minutes. The supernatant is discarded, and the cell pellet is resuspended in 1 ml of Cryostor CS10 (10% DMSO) in a cryovial. The vial is then frozen in a CoolCell controlled-rate freezing container in a -80°C freezer. After 24 hours, vials of SVF are transferred to a storage box stored in a liquid nitrogen freezer until the day of injection 6 months later.
Thaw
Once the 6-month mark of the study is completed and if the control patient is still having significant knee pain and not significantly improved by the LR injection, they may elect to be unblinded and receive SVF injection performed under ultrasound guidance. On the day of administration, cells are thawed using a 37°C water bath or ThawSTAR = Automated Cell Thawing System. Once thawed, cells are transferred to a conical tube and washed 1X with LR solution to remove DMSO. Cells are resuspended in 10 ml LR and pulled into a 10-20 ml syringe for injection. Pre-release testing is performed for cell count, viability, gram stain and endotoxin. (Table 1) If viability is below 70% or any contamination is present, the product will not be administered to the patient which is a standard precaution in many IND cell therapy trials.
Results
The pre-release test results for the first three control patient SVF products were conducted after thawing and before administration (Table 1). These tests are essential to ensure the quality and safety of the product before it was administered to the patient. The thawed SVF product underwent rigorous analysis, including assessments for sterility, viability, and cell count. These tests aimed to verify that the product maintained its integrity and functionality throughout the freezing and thawing process. The results of these pre-release tests provided crucial information for healthcare professionals to make informed decisions regarding the administration of the SVF product, ensuring the best possible outcome for the control patient.
Table 1: Pre-release test results for control patient SVF product.
|
Control Patient |
Cell Dose |
Viability (%) |
Endotoxin |
Gram Stain |
Culture |
|
1 |
3.21 x 106 |
73 |
Neg |
Neg |
Neg |
|
2 |
5.15 x 106 |
83 |
Neg |
Neg |
Neg |
|
3 |
3.45 x 106 |
93 |
Neg |
Neg |
Neg |
Discussion
In this report, we demonstrate early success of a clinical trial design investigating an autologous orthobiologic treatment that allows for a placebo control arm without needing to perform an unnecessary liposuction procedure that would potentially be of no benefit to the study patient. We hypothesize that providing placebo group patients the opportunity to receive their cells at the conclusion of their participation in the comparison portion of the study and all efficacy outcome measures have been completed will aid in subject recruitment for the placebo arm. We demonstrated the ability to freeze and thaw the investigational product successfully in subjects treated in the placebo arm thus far.
The randomized controlled trial is commonly employed when attempting to determine efficacy of a novel therapeutic intervention in clinical trial research. However, there have been concerns regarding practical or ethical options to include a placebo arm within the RCT especially if there can be no benefit to the patient. In addition, in the context of research involving novel cellular therapy, recruitment for such studies is difficult if potential subjects have concerns that they will not receive the study drug.
The purpose of this study design is to assist with subject recruitment. Such a design allows for investigators to compare treatment to placebo control while still being able to adequately recruit subjects for the trial. Of note, because the design is primarily a safety and feasibility trial, no crossover analysis of secondary efficacy outcomes will be performed. This is because the study is designed to test manufacturing of SVF at the bedside while also investigating the safety of the resulting product. Efficacy of the SVF is a secondary outcome of this trial, but in order to adequately assess that efficacy, the study subjects must be blinded. In order to ethically blind the treatment subjects, there must be a random chance to receive the placebo treatment. We did not design the study to investigate the outcomes of those placebo subjects because we could not guarantee the ability to preserve the SVF for the entire 6 months without potential for some failures in preservation and thawing. Nor can we be certain the thawed product is as biologically active as the fresh SVF. This would create statistical comparison problems between the two groups and for this reason, a cross over design whereby both groups undergo a single liposuction procedure at the time of enrollment but one group gets their product stored and then crosses over to a treatment regimen to assess that treatment efficacy is not feasible and at the very least would require many more subjects to account for the expected variability in the preserved SVF. The purpose of the preservation is to provide placebo arm subjects some compensation and some potential for therapeutic benefit in exchange for their willingness to undergo a liposuction procedure which otherwise would then have been deemed an unacceptable risk if the liposuction procedure was performed only for the patient to receive a LR injection.
SVF has previously been studied for osteoarthritis treatments5,23,27. Despite success thus far, a few characteristics and limitations to such study designs bear mentioning. Successful delivery of stored product back to the patient at the 6 month mark is subject to some pitfalls. The minimum, safe storage temperature for a sample must be maintained throughout the cryo-chain to avoid any warming or thawing that could lead to viability loss or weakened function of the product. Investigators must have access to collaborators with expertise in cell therapy which requires industry collaboration or a university hospital setting. Even with such expertise, there is an expected cell viability deterioration, which if less than 70%, falls below conventional agreed upon cell therapy convention28. For this reason, a thorough informed consent discussion at the time of study enrollment needs to include the possibility that despite study design with preservation of the patient’s cells, they may not receive the autologous manufactured product. Additionally, even with strict sterile technique, product contamination is possible and if the product fails regulatory required pre-release testing (gram stain, endotoxin, culture, etc.) this too may prevent the patient from receiving the intended therapeutic product.
These typical FDA in-process product testing and release criteria (cell count, viability, and sterility) are integral to ensure quality and consistency of the product. However, such parameters may currently be difficult to manage for first generation orthobiologic procedures such as autologous adipose tissue applications like the SVF utilized in this study design. It is important to note that SVF is characterized as a heterogenous cell population consisting of progenitor cells, both hematopoietic and mesenchymal, macrophages, monocytes, T cells, B cells, endothelial cells, and some known cytokines (IL1-ra, VEGF(A), IL-6, IL-8, CCL7/MCP3, GM-CSF, and HGF)5,29,30. While current US code of federal regulations (CFR 1271) do not require such pre-release testing for some of these orthobiologics, clinical trials needed for scientific validation certainly will. As a result, study investigators and clinical providers alike will need to familiarize themselves with such concepts to successfully navigate the regenerative therapeutic product development pathway. Finally, orthobiologic providers and product developers hope to engage the FDA regarding the development of safe chemistry, manufacturing and control processes designed to accommodate imperfections in manufacturing and preservation/thaw process but that are not as rigorous as Good Manufacturing Practice (GMP) standards.
Conclusion
Novel orthobiologic therapies will require novel product development and clinical study tools. Rigorous randomized controlled trials investigating treatments for osteoarthritis must account for the placebo response and investigators must consider the unique elements of future study designs to yield the desired translation of orthobiologics into routine clinical practice. The study of 1st generation orthobiologics can benefit from the utilization of available advanced biomanufacturing tools such as cell preservation and thaw strategies as presented here.
List of Abbreviations
BMAC: Bone marrow aspirate concentrate
DMSO: Dimethyl sulfoxide
FDA: Food & Drug Administration
GMP: Good manufacturing practice
HCTL: Human Cell Therapy Lab
IND: Investigational New Drug Applications
LR: Lactated ringer
MFAT: Microfragmented adipose tissue
Neg: Negative
OA: Osteoarthritis
PRP: Platelet rich plasma
RCT: Randomized controlled trial
SOP: Standard operating procedures
SVF: Stromal vascular fraction
Declarations
Ethics Approval and Consent to Participate
This study was approved by the Mayo Clinic Institutional Review Board (IRB) (Protocol # 18-006909). All methods were carried out in accordance with the approved Mayo Clinic IRB protocol. Informed consent was obtained from all subjects prior to study participation.
Availability of Data and Materials
The datasets supporting the conclusion of this trial will be available from the corresponding author on reasonable request.
Competing Interest
The authors declare they have no competing interests.
Funding
None
Authors’ Contributions
SH wrote the first draft of the manuscript, designed figure, and edited the manuscript. JA wrote and edited the manuscript. SS conceptualized the design of study, wrote, and edited the manuscript. MH Conceptualized the study design and edited portions of the manuscript. All authors contributed to the revision of the manuscript.
Acknowledgements
This investigation is supported by the Louis V. Gerstner, Jr. Fund at Vanguard Charitable.
References
- Neogi T. The epidemiology and impact of pain in osteoarthritis. Osteoarthritis Cartilage. 2013; 21(9): 1145-53. doi:10.1016/j.joca.2013.03.018
- Kloppenburg M, Berenbaum F. Osteoarthritis year in review 2019: epidemiology and therapy. Osteoarthritis Cartilage. 2020; 28(3): 242-248. doi:10.1016/j.joca.2020.01.002
- Filardo G, Previtali D, Napoli F, et al. PRP Injections for the Treatment of Knee Osteoarthritis: A Meta-Analysis of Randomized Controlled Trials. Cartilage. 2021; 13(1_suppl): 364S-375S. doi:10.1177/1947603520931170
- Fitzpatrick J, Bulsara M, Zheng MH. The Effectiveness of Platelet-Rich Plasma in the Treatment of Tendinopathy: A Meta-analysis of Randomized Controlled Clinical Trials. Am J Sports Med. 2017; 45(1): 226-233. doi:10.1177/0363546516643716
- Garza JR, Campbell RE, Tjoumakaris FP, et al. Clinical Efficacy of Intra-articular Mesenchymal Stromal Cells for the Treatment of Knee Osteoarthritis: A Double-Blinded Prospective Randomized Controlled Clinical Trial. Am J Sports Med. 2020; 48(3): 588-598. doi:10.1177/0363546519899923
- Gobbi A, Dallo I, Rogers C, et al. Two-year clinical outcomes of autologous microfragmented adipose tissue in elderly patients with knee osteoarthritis: a multi-centric, international study. Int Orthop. 2021; 45(5): 1179-1188. doi:10.1007/s00264-021-04947-0
- Mautner K, Bowers R, Easley K, et al. Functional Outcomes Following Microfragmented Adipose Tissue Versus Bone Marrow Aspirate Concentrate Injections for Symptomatic Knee Osteoarthritis. Stem Cells Transl Med. 2019; 8(11): 1149-1156. doi:10.1002/sctm.18-0285
- Anz AW, Hubbard R, Rendos NK, et al. Bone Marrow Aspirate Concentrate Is Equivalent to Platelet-Rich Plasma for the Treatment of Knee Osteoarthritis at 1 Year: A Prospective, Randomized Trial. Orthop J Sports Med. 2020; 8(2): 2325967119900958. doi:10.1177/2325967119900958
- Shapiro SA, Kazmerchak SE, Heckman MG, et al. A Prospective, Single-Blind, Placebo-Controlled Trial of Bone Marrow Aspirate Concentrate for Knee Osteoarthritis. Am J Sports Med. 2017; 45(1): 82-90. doi:10.1177/0363546516662455
- Filardo G, Tschon M, Perdisa F, et al. Micro-fragmentation is a valid alternative to cell expansion and enzymatic digestion of adipose tissue for the treatment of knee osteoarthritis: a comparative preclinical study. Knee Surg Sports Traumatol Arthrosc. 2022; 30(3): 773-781. doi:10.1007/s00167-020-06373-y
- Dai WL, Zhou AG, Zhang H, et al. Efficacy of Platelet-Rich Plasma in the Treatment of Knee Osteoarthritis: A Meta-analysis of Randomized Controlled Trials. Arthroscopy. 2017; 33(3): 659-670 e1. doi:10.1016/j.arthro.2016.09.024
- Bannuru RR, Schmid CH, Kent DM, et al. Comparative effectiveness of pharmacologic interventions for knee osteoarthritis: a systematic review and network meta-analysis. Ann Intern Med. 2015; 162(1): 46-54. doi:10.7326/M14-1231
- Marx RG, Wilson SM, Swiontkowski MF. Updating the assignment of levels of evidence. J Bone Joint Surg Am. 2015; 97(1): 1-2. doi:10.2106/JBJS.N.01112
- Fregni F, Imamura M, Chien HF, et al. Challenges and recommendations for placebo controls in randomized trials in physical and rehabilitation medicine: a report of the international placebo symposium working group. Am J Phys Med Rehabil. 2010; 89(2): 160-72. doi:10.1097/PHM.0b013e3181bc0bbd
- Vase L. Can insights from placebo and nocebo mechanisms studies improve the randomized controlled trial? Scand J Pain. 2020; 20(3): 451-467. doi:10.1515/sjpain-2019-0183
- Previtali D, Merli G, Di Laura Frattura G, et al. The Long-Lasting Effects of "Placebo Injections" in Knee Osteoarthritis: A Meta-Analysis. Cartilage. 2021; 13(1_suppl): 185S-196S. doi:10.1177/1947603520906597
- Stern PJ. Hattage. J Bone Joint Surg Am. 2007; 89(12): 2803-9. doi:10.2106/JBJS.G.01208
- Bannuru RR. Editorial Commentary: Intra-Articular Injections for Painful Knee Osteoarthritis: What Is the Current Treatment Paradigm? Arthroscopy. 2021; 37(1): 307-308. doi:10.1016/j.arthro.2020.09.031
- Zanata F, Bowles A, Frazier T, et al. Effect of Cryopreservation on Human Adipose Tissue and Isolated Stromal Vascular Fraction Cells: In Vitro and In Vivo Analyses. Plast Reconstr Surg. 2018; 141(2): 232e-243e. doi:10.1097/PRS.0000000000004030
- Bateman ME, Strong AL, Gimble JM, et al. Concise Review: Using Fat to Fight Disease: A Systematic Review of Nonhomologous Adipose-Derived Stromal/Stem Cell Therapies. Stem Cells. 2018; 36(9): 1311-1328. doi:10.1002/stem.2847
- Suh A, Pham A, Cress MJ, et al. Adipose-derived cellular and cell-derived regenerative therapies in dermatology and aesthetic rejuvenation. Ageing Res Rev. 2019; 54: 100933. doi:10.1016/j.arr.2019.100933
- Yang WT, Ke CY, Yeh KT, et al. Stromal-vascular fraction and adipose-derived stem cell therapies improve cartilage regeneration in osteoarthritis-induced rats. Sci Rep. 2022; 12(1): 2828. doi:10.1038/s41598-022-06892-3
- Garza JR SMD, Palomera T, Dumanian GA, et al. Use of autologous adipose-derived stromal vascular fraction to treat osteoarthritis of the knee: a feasibility and safety study. J Regen Med. 2015.
- Chan AW, Tetzlaff JM, Altman DG, et al. SPIRIT 2013 statement: defining standard protocol items for clinical trials. Ann Intern Med. 2013; 158(3): 200-7. doi:10.7326/0003-4819-158-3-201302050-00583
- Chan AW, Tetzlaff JM, Gotzsche PC, et al. SPIRIT 2013 explanation and elaboration: guidance for protocols of clinical trials. BMJ. 2013; 346: e7586. doi:10.1136/bmj.e7586
- Pocock SJ, Simon R. Sequential treatment assignment with balancing for prognostic factors in the controlled clinical trial. Biometrics. 1975; 31(1): 103-15.
- Hong Z, Chen J, Zhang S, et al. Intra-articular injection of autologous adipose-derived stromal vascular fractions for knee osteoarthritis: a double-blind randomized self-controlled trial. Int Orthop. 2019; 43(5): 1123-1134. doi:10.1007/s00264-018-4099-0
- Content and Review of Chemistry, Manufacturing, and Control (CMC) Information for Human Somatic Cell Therapy Investigational New Drug Applications (INDs) Guidance for FDA Reviewers and Sponsors. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/content-and-review-chemistry-manufacturing-and-control-cmc-information-human-somatic-cell-therapy
- Lin K, Matsubara Y, Masuda Y, et al. Characterization of adipose tissue-derived cells isolated with the Celution system. Cytotherapy. 2008; 10(4): 417-26. doi:10.1080/14653240801982979
- Polancec D, Zenic L, Hudetz D, et al. Immunophenotyping of a Stromal Vascular Fraction from Microfragmented Lipoaspirate Used in Osteoarthritis Cartilage Treatment and Its Lipoaspirate Counterpart. Genes (Basel). 2019; 10(6): 474. doi:10.3390/genes10060474
