Therapeutic Arterial Embolization in Patients with Shoulder Adhesive Capsulitis: A Systematic Review
Amina Ait Belmahjoub Aamre MD1, Sergio Barroso Rosa FEBOT PhD1,2*
1Department of Medical and Surgical Sciences, Universidad de Las Palmas de Gran Canaria, Las Palmas de Gran Canaria, Spain
2Department of Orthopaedics, Complejo Hospitalario Universitario Insular Materno Infantil de Gran Canaria, Las Palmas de Gran Canaria, Spain
Abstract
Introduction: Currently there are limited options for shoulder adhesive capsulitis treatment, some of which do not have sufficient backing scientific evidence. This entails a relevant setback for patients, especially in severe or refractory cases. In recent years, remote arterial embolization therapies have demonstrated usefulness in the management of diverse musculoskeletal conditions. This systematic review examines the therapeutic role of arterial embolization in patients with shoulder adhesive capsulitis.
Methods: A systematic review of articles published to date was performed, according to the methodology in the Cochrane Systematic Reviews Manual (MECIR) and the PRISMA checklists. PubMed, Scopus, Google Scholar, three other databases and three trial registries were examined for relevant studies. The ROBINS-I tool was used for quality assessment of included studies.
Results: Ninety four potential articles were found, seven of which were included. In the selected studies, arterial embolization was carried out in 127 patients, 113 of whom had abnormal vessels. In all studies, a reduction in pain and improvement in mobility was observed in less than six months after the procedure. There were no major adverse effects or recurrent symptoms reported. Due to large data heterogeneity, a meta-analysis was not performed. Three literature reviews were also included as part of the background discussion.
Conclusions: Arterial embolization is an effective and safe treatment option in patients with shoulder adhesive capsulitis, resulting in reduced pain and restored joint function. Controlled randomized trials are required to evaluate the attributable effect of the technique to the reported clinical improvement.
Introduction
Osteoarticular conditions are a prevalent medical and social problem in modern societies. Specifically, shoulder adhesive capsulitis (SAC), also known as "frozen shoulder", is a fairly common disorder in our setting with an estimated prevalence of around 2%-5% in the general population1. This condition is more frequent in women and presents a peak incidence between 40-60 years1,2. Associated predisposing factors such as diabetes, hypothyroidism, hyperthyroidism, hypoadrenalism and other hormonal imbalances have been identified in secondary cases1. SAC can also occur as a secondary joint impairment after fractures or trauma to the shoulder, rotator cuff injuries or surgery3.
SAC is an inflammatory idiopathic process with an unclear origin. It is often thought to occur as a result of soft tissue damage, which has been pointed out as a trigger for a complex cascade of growth factors and cytokines, leading to increased extracellular matrix turnover and fibroblast activation with collagen deposition and reduced extracellular matrix degradation1,4. Furthermore, recent angiographic studies have observed an abnormal neoangiogenesis around the glenohumeral joint described as "burning sign" or “tumor blush” that may explain, along with the other findings, the origin of regional pain5,6. As a sum, a chronic inflammatory response with fibroblastic proliferation, angiogenesis, synovitis and thickening of the joint capsule is produced, favoring stimulation of adjacent pain receptors, thus explaining the functional limitation and pain.
SAC has been considered self-limiting, with three traditionally described phases: an initial freezing or painful phase, a frozen or stiff phase and a final phase of thawing or recovery. Unfortunately, these phases can often last up to 36 months1,7-9. Standard management of SAC involves oral painkillers, local injections, physiotherapy and surgical release in refractory cases, often with suboptimal results3,4.
In recent years, trans-arterial embolization (TAE) in patients with SAC arises as a therapeutic alternative, with the aim of blocking arterial flow theoretically responsible for the inflammatory and fibrotic status. The objective of this review is to analyze the available literature reporting clinical and functional results of TAE as a therapeutic option for SAC, and whether it can be recommended as an elective treatment, facilitating decision-making in the therapeutic management of this condition.
Methods
This systematic review was performed following the methodology described in the Cochrane Systematic Reviews Manual (MECIR) and the PRISMA (preferred reporting items for systematic review and meta-analysis) checklists10,11. Ethics committee or institutional review board approval was not required. The review was registered at the International prospective register of systematic reviews – PROSPERO – in June 2022 (registration number CRD42022337382). A meta-analysis was not plausible due to the heterogeneity between studies, variable outcome measures and highly biased study types.
Eligibility criteria
Studies disclosing clinical outcomes of TAE to treat primary or secondary SAC in adult patients were eligible for inclusion. The exclusion criteria were having atherosclerosis, bursitis, neoplasm, infections, rheumatoid arthritis, spondylarthritis or microcrystalline rheumatism, irreversible coagulopathy, complete rotator cuff tear and short life expectancy.
Search strategy
A structured search was performed in the following databases and trial registers: PubMed, Google Scholar, ScienceDirect, Scopus, LILACS, CENTRAL, ClinicalTrials.gov and ICTPR. Other databases linked to the authors affiliations were also explored. Scientific publications were searched from inception to June 2022. Studies were identified using the MeSH search terms: “Embolization AND adhesive capsulitis” and “Frozen shoulder AND embolization”, as well as combination of these terms. Same search terms were used in Spanish and French languages. The “human” filter was used when presented.
Study selection
Study screening and selection were performed by two authors independently (AA and SB). In case of discrepancy, a senior vascular radiologist acted as a referee to determine study inclusion or rejection. Study selection flowchart is summarized in Figure 1.
Figure 1: PRISMA flow diagram of literature selection.
Risk of bias analysis
The ROBINS-I risk of bias tool to assess non-randomized studies of interventions was used to evaluate the quality of the selected studies12. Analysis was performed independently by two authors, AA and SB. To graphically present the risk of bias in the studies included, the Robins online visualization tool was used13. Case reports were not scored, as the risk of bias is systematically considered critical for such type of article.
Data presentation
Due to heterogeneity, multiple outcome measures and highly biased study types, conducting a meta-analysis was not possible. To enable comparison of the visual analogue scale (VAS) between studies, conversion was made to VAS 0–100 mm in those studies reporting VAS in a 0–10 cm scale14. A p value < 0.05 was considered statistically significant.
Results
In the initial search, a total of 5346 references were identified, ending up with a total of 94 articles selected for the review (Figure 1). After removal of duplicates and abstract screening, 12 studies were selected to be included in the review. This review includes prospective and retrospective non-randomized interventional studies, as well as one case report, one abstract and three systematic reviews as part of the background discussion. Seven studies were eligible in total for the qualitative synthesis. All studies were published between 2014 - 2022.
Patient characteristics
Selected articles gathered data from a total of 127 patients, ( age range 27 – 69) 79.5% were women and 20.5% men. Affected side was approximately equal; 53% of right shoulder, 47% left. Body mass index (BMI) was only reported in one study conducted in USA15; the mean body mass index was 29.8 kg/m2 (range 20.04–41.1 kg/m2). 36% of the subjects in that study were considered obese (body mass index range, 30–34 kg/m2). Presence of diabetic patients was specified in three studies16-18. The characteristics of the patients included in this literature review can be seen in Table 1.
Table 1: Characteristics of the studies included in the Systematic Review
|
Study |
Year |
Country |
Type of study |
n |
Sex (F/M) |
Age |
Side (R/L) |
AE |
Lost to FU |
FU time |
|
Okuno et al. |
2014 |
Japan |
Prospective non-randomized |
7 |
5/2 |
50 |
3/4 |
No AE |
0 |
6 - 16 (10) |
|
Okuno et al. |
2016 |
Japan |
Prospective non-randomized |
25 |
16/9 |
53,8 |
16/9 |
10 (minor) |
1 |
30 - 44 (36.1) |
|
Férnandez-Martínez et al. |
2020 |
Spain |
Prospective non-randomized |
40 |
35/5 |
50 |
20/20 |
2 (minor) |
0 |
12 - 48 (21.2) |
|
Ciampi-Dopazo et al. |
2020 |
Spain |
Prospective non-randomized |
9 |
6/3 |
48 |
4/5 |
No AE |
0 |
6 |
|
Sajan et al. |
2021 |
USA |
Case report |
1 |
1/0 |
40 |
0/1 |
No AE |
0 |
6 |
|
Férnandez-Martínez et al. |
2021 |
Spain |
Prospective non-randomized |
25 |
20/5 |
49 |
NR |
6 (minor) |
0 |
6 - 41 (17) |
|
Bagla et al. |
2022 |
USA |
Prospective non-randomized |
20 |
18/2 |
51 |
11/9 |
9 (minor) |
8 |
6 |
Age: mean age
AE: adverse events (n)
Lost to FU: patients lost during follow-up (n)
FU time: time in months, range and (mean)
All studies consisted of subjects with idiopathic frozen shoulder except Fernández-Martínez et al.19 which excluded patients with idiopathic shoulder stiffness; in this study the aetiology of secondary stiff shoulder was postoperative in 56% and post-traumatic in the remaining 44%. 42.8% of patients in this study had undergone surgery at least twice.
All studies included patients with at least ≥ three months of shoulder pain before TAE treatment, except Bagla et al. which included patients with pain refractory to at least 30 days of conservative treatment15. In all studies, the diagnosis of adhesive capsulitis was based in clinical findings elicited by physical examination. Imaging diagnostic studies were performed in all studies except in both studies by Fernández-Martínez et al.17,19, in which radiological examination was not mentioned. MRI scan was performed in four studies15,17,19,20. Ultrasonography was performed in three articles to confirm the diagnosis16 or as an alternative to MRI scan due to claustrophobia or other disabling conditions17,20.
TAE procedure
All studies included the use of local anesthesia except for one15 which did not report the use of anesthetics. Fernández-Martínez et al.19 also described the application of topical ice packs 20 min before and after the procedure to avoid unwanted migration of the embolic agent. TAE was performed through radial16,20,21 or femoral access17,19 exclusively while other authors employed both alternatives15,18. Most of the studies used a resorbable embolic agent, IMP/CS (Imipenem/Cilastatin Sodium), except for Sajan et al.21 and Bagla et al.15 where they used polyethyleneglycol (PEG) microspheres (HydroPearl – Terumo). Embolic agent volume was documented by Fernández-Martínez et al.17 and Okuno et al.20, with mean embolic agent amount of 1.6 ml (range 0.2–3.1 ml) and 0.94 ml (range 0.6 – 1.6 ml) applied per procedure, respectively. Remaining studies did not state the embolic volume used but most of them disclosed performing TAE administering aliquots of 0.2 ml of the embolic solution. Fernández-Martínez et al.17, Okuno et al.20 and Bagla et al.15 reported mean time of the embolization procedure as 48 ± 17.2 min; 35 ± 6 min (range, 29-45 min) with a mean fluoroscopy time of 9.8 ± 2.1 min (range 8.2-13.3 min) and mean estimated dose-area product of 11.7 ± 1.2 Gy/cm2 (range 10.1-13.1 Gy/cm2); and 69.3 min ± 26.9 with an average fluoroscopy time of 28.6 minutes ± 22.5 and administered reference air kerma of 73.1 mGy ± 39.2 respectively. The number of arteries embolized varied from 1-6 arteries per TAE procedure. Table 2 summarizes the data about TAE procedure in the different studies.
Table 2: Summary of procedure technical data
|
Tumor blush (n)
|
EA
|
LA
|
Access
|
Arteries
|
Embolic volume
|
Time
|
Discharge
|
|
|
Okuno et al. 2014 |
7 |
IMP/CS |
Yes |
Radial |
1 - 4 |
0.6 - 1.6 |
35 ± 6 |
Same day (2 hours after TAE) |
|
Okuno et al. 2016 |
25 |
IMP/CS |
Yes |
Radial |
≥ 1 |
NR |
NR |
Same day |
|
Férnandez-Martínez et al. 2020 |
31 |
IMP/CS |
Yes |
Femoral |
NR |
1.6 |
48 ± 17.2 |
Same day (8 hours after TAE) |
|
Ciampi-Dopazo et al. 2020 |
9 |
IMP/CS |
Yes |
Radial and femoral |
1 - 3. |
NR |
NR |
Next day |
|
Sajan et al. 2021 |
1 |
PEG |
Yes |
Radial |
6 |
NR |
NR |
Same day (4 hours after TAE) |
|
Férnandez-Martínez et al. 2022 |
20 |
IMP/CS |
Yes |
Femoral |
≥ 1 |
NR |
NR |
Same day (8 hours after TAE) |
|
Bagla et al. 2022 |
20 |
PEG |
NR |
Radial and femoral |
4 |
NR |
69.3 ± 26.9 |
Same day |
EA: embolic agent
LA: local anesthesia
Arteries: arteries embolized per patient (n)
Embolic volume: mls
Time: procedure duration in minutes (mean ± SD)
NR= not reported
IMP/CS = Imipenem Cilastatin/Sodium
PEG = Polyethylene Glycol microspheres (HydroPearl – Terumo)
Angiography was performed prior to TAE in all the studies, with the objective of searching abnormal neovessels. These are generally described as hyperemic areas, identified by a described ‘‘tumor-blush’’ type of enhancement often accompanied by early venous drainage. Out of the 127 patients, abnormal neovessels were found in 113, which makes this phenomenon present in 89% of the subjects included in this review. In the cases where patients did not present a “tumor blush”17,19, target vessels were selected deployment of contrast in those arteries corresponding to the painful areas previously observed on physical examination. In these cases, patients referred an “evoked pain” that included pain, itching, or heat sensation during contrast injection, which reproduced in the same way when a blush enhancement area was embolized in most patients. This occurrence was used to identify arteries responsible for the patients’ pain17. The most frequent abnormal arteries found in the studies where the thoracoacromial artery and the anterior circumflex humeral artery; others were the coracoid branch, the posterior circumflex humeral, the circumflex scapular, and the suprascapular.
Four studies mentioned that TAE procedures were performed by radiologists with at least five years of experience in embolization procedures15,17,19,20.
All patients were discharged the same day two to eight hours after TAE, except Ciampi-Dopazo et al.18 where patients were discharged hospital the day after the procedure. Most authors recommended relative rest for the rest of the day and all patients were asked to move the shoulder one day after TAE.
Follow-up
All the studies included follow-ups at 1, 3 and 6 months. Final follow-up time ranged from 6 to 48 months. Okuno et al.16 and Bagla et al.15 were the only studies that reported patients lost to follow-up, with one and eight patients respectively, the latter mainly due to COVID-19 pandemic restrictions. One patient was excluded after taking corticosteroid injections before three months follow up15.
Outcome measures
Outcome measures systems were variable in the different studies included, yet pain, which was the primary outcome in most of the studies, was reported using the VAS in all of them. Some studies included variables like night-time pain, pain at rest or during activity22. Figure 2 illustrates pain reduction after TAE. There was a decrease in post-treatment pain in the totality of the studies, which resulted more significant right after the procedure and three months later.
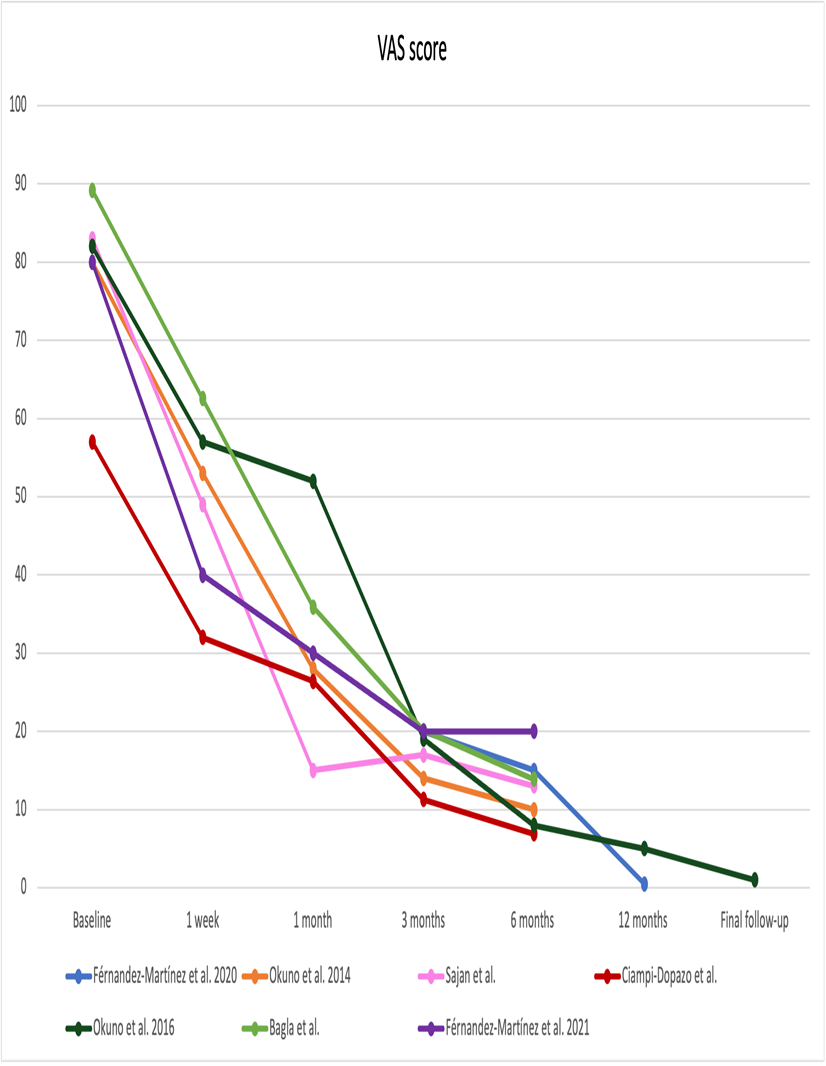
Figure 2: Pain records after trans-arterial embolization procedure. Pain was measured with the visual analog scale (VAS).
Range of movement (ROM) variation was recorded in all studies except Sajan et al.21 and Bagla et al.15; both studies used SANE (Single Assessment Numeric Evaluation) instead, which is a patient reported single-item global outcome measure. There was a marked improvement in SANE scores for both studies after six months. ROM was evaluated heterogeneously in the studies, measuring flexion, abduction, and internal/external rotation. However, they all evaluated shoulder flexion. The evolution of ROM in shoulder flexion is shown in Figure 3.
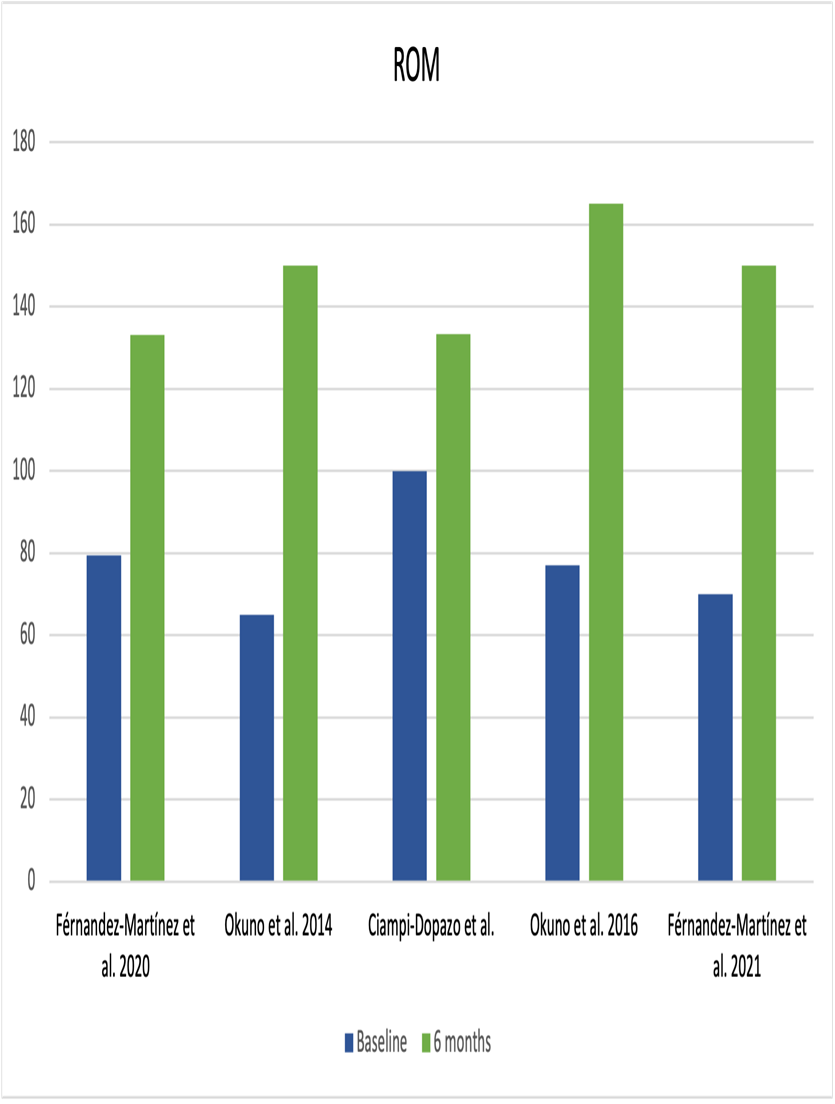
Figure 3: Shoulder range of movement (ROM). A shoulder flexion increase can be seen from baseline to six months after TAE.
Ciampi-Dopazo et al.18, unlike the rest of the studies, based their inclusion criteria on ranges of mobility (ROM) mainly, not on pain scores which made the baseline scores for pain at rest low; thus, there was little room for improvement, and this may explain the lack of statistically significant improvement. In addition, they reported that the two patients in their series who had diabetes (22.2% of cases) showed the worst response to treatment in terms of ROM, which brought down the overall means.
To assess shoulder functionality, ASES scores (American Shoulder and Elbow Surgeons Scale) and the Quick DASH (Disabilities of the Arm, Shoulder, and Hand) questionnaire were used by four15,16,20,21 and one studies18 respectively. In all the studies that measured shoulder function, an improvement over time was observed, being significant at six months after TAE.
Physical therapy and use of analgesic medication before TAE were described in all studies. Corticosteroid infiltrations were also reported as a treatment before TAE in most studies, except for two. Okuno et al.16 reported a decrease from 84% in physical therapy need, 80% anti-inflammatory drugs use and 72% corticosteroid injections to 0% in the final follow up. Bagla et al.15 also documented no analgesic use at 3- and 6-months follow-up. Fernández-Martínez et al.17,19 in both of their studies reported similar reduction in the use of oral analgesics. In the latter study, a corticosteroid infiltration was performed in one patient four months after TAE, and 96% of patients began physical therapy two weeks after embolization, except for one patient, in whom it was not necessary. Furthermore, surgery was performed in four patients (16%); arthroscopic capsular release, in one patient because of no recovery and in two patients because decreased pain was achieved but not the target ROM. In the remaining patient, arthroscopic repair of the supraspinatus tendon was performed. In the study of Ciampi-Dopazo et al.18, intensive physical therapy (30 minutes of physical therapy performed by physiotherapist (ROM) and electrotherapy (pain), five days per week plus home exercise two or three times every day) before TAE was an inclusion criterion. After treatment, rehabilitative therapy was carried out in all patients per protocol.
Adverse events
Adverse events were reported in four studies, all of them being minor mild side effects. Fernández-Martínez et al.17,19 reported groin discomfort secondary to arterial puncture site hematoma, transitory change in skin color, and painless temporary erythema with an average duration of 49.5 min (range 22-102 min). Bagla et al.15 also reported skin discoloration in 41.1% of patients, and itchiness in 11.8% of the patients. Okuno et al.16 informed about other mild adverse events such as strong evoked pain during procedure which resolved during procedure or 1h after, radial artery spam which resolved without treatment, puncture site pain for one week and fever for one night. No major adverse events were informed in any study. No patients reported peripheral paresthesia, muscle weakness, or shoulder instability during the follow-up period. Table 3 shows the adverse effects per study.
Table 3: Summary of reported adverse effects
|
Adverse events, n (%) |
|||||||||
|
Access site hematoma/pain |
Skin discoloration |
Itchiness |
Temporary erithema |
Strong evoked pain during process |
Fever |
Access artery spasm |
|||
|
Okuno et al. 2014 |
- |
- |
- |
- |
- |
- |
- |
||
|
Okuno et al. 2016 |
1 (4%) |
- |
- |
- |
5 (20%) |
1(4%) |
2 (8%) |
||
|
Férnandez-Martínez et al. 2020 |
2 (5%) |
- |
- |
- |
- |
- |
- |
||
|
Ciampi-Dopazo et al. 2020 |
- |
- |
- |
- |
- |
- |
- |
||
|
Sajan et al. 2021 |
- |
- |
- |
- |
- |
- |
- |
||
|
Férnandez-Martínez et al. 2022 |
- |
2 (8%) |
- |
2 (8%) |
- |
- |
- |
||
|
Bagla et al. 2022 |
- |
7 (35%) |
2 (10%) |
- |
- |
- |
- |
||
Risk of bias in studies
The ROBINS-I risk of bias tool was used to assess the quality of the included studies12. Assessment was performed independently by two authors (AA and SB): there were no disagreements between the authors. Graphic representation of the quality of the studies evaluation can be seen in Figure 4. The overall risk of bias is moderate in most (71.4%) of the studies included. Bias due to different bias domains was assessed following the guidelines of our tool of choice.
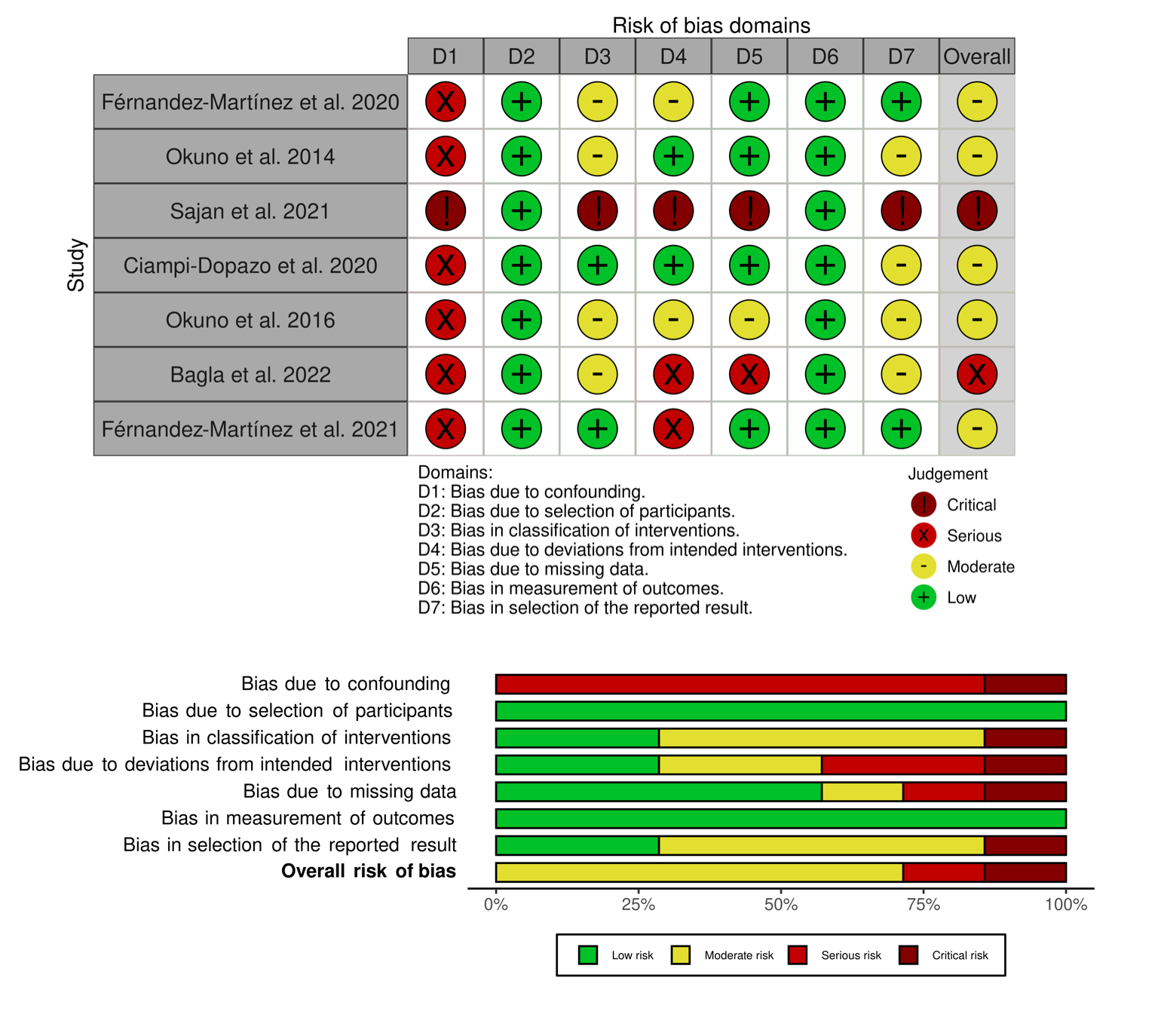
Figure 4: Risk of bias assessment with ROBINS-I tool. Traffic-light plot and a summary plot were used to graphically represent the results.
Discussion
TAE is a relatively recent practice in the treatment of inflammatory musculoskeletal conditions. Firstly reported in 2013, Okuno et al. described this technique to treat varied enthesopathies such as patellar tendon or lateral epicondylitis23. Subsequently, TAE has been proposed for other musculoskeletal entities, mainly in cases non-responding to conventional treatments15-21,24-29. The application of this technique relies on two non-discriminatory theoretical frames: a) the presence of angiographic hypervascularization suggests continuous inflammation thus occlusion of these abnormal vessels may decrease the influx of inflammatory cells and proinflammatory cytokines, reducing the inflammatory process16,30, or b) hypervascularization causes stimulation of adjacent unmyelinated sensory nerve endings and embolization could reduce this stimulation decreasing pain16,31,32.
Numerous investigations have described abnormal hypervascularity in patients with SAC, notably within the capsule, using enhanced MRI5,33,34, surgical observation1,2,22,34, and histologic examination4,22. In the studies included in our review, angiography showed neovessels in 89% of the patients, which suggest hypervascularization plays an important role in the pathogenesis of adhesive capsulitis. To our knowledge it is unknown it this phenomenon could be present in asymptomatic patients, too.
In all the studies reviewed, pain was reduced significantly in the first six months and there were no signs of recurrence of symptoms or relapse in the final follow-ups, which was 48 months in the group longer observed. Similarly, mobility and shoulder function improved in all the included studies where it was assessed in terms of ROM; SANE or ASES. Some of the studies included prescribed ROM exercises in all their patients before and after the procedure, which makes it difficult to measure the owning efficacy of TAE therapy in the final outcome. This has been considered to be a very important confounding factor by the authors of the present review. In addition, there was a clear reduction in analgesics use after TAE.
The incidence of adverse events due to TAE can vary from 0.4% to 12%, ranging from minor complications which do not require medical management, to major complications such as permanent sequelae or even death 35. In our review, all the reported events were minor and of temporary nature. There were no major complications requiring hospital admission or emergency medical care. The adverse events may have been related to non-target embolization, resulting from the inadvertent embolization of cutaneous arteries (which may be difficult to avoid, given the size and location of these branch vessels). Bagla et al.15 observed that a possible solution to this could be the use of larger embolic particles that would prevent unwanted embolization of these thinner distal cutaneous arterial branches. It is unknown whether increasing the size of the embolic particles would affect the clinical efficacy of the procedure. However, as previously exposed in the results section, using different types of embolization materials did not make a significant change in the overall results or in the adverse effects reported. Having said that, study samples are small and heterogenic, therefore robust comparison among the properties of different embolic agents cannot be obtained.
In the study Fernández-Martínez et al.19 with secondary SAC patients, the results do not differ greatly from those of idiopathic SAC, which may lead us to believe that TAE may be equally effective in both idiopathic and secondary SAC. On the other hand, one study mentioned that diabetic patients showed a worse response to TAE and had overall worse results in terms of ROM, but this finding may lack statistical significance18. Nevertheless, further research with larger series is warranted to further interpret these findings.
Currently there is no formal consensus on the optimal therapeutic management for SAC. All the subjects included in this review had previously received treatment before TAE, either with oral analgesia, physiotherapy, corticosteroid infiltrations, or a combination of these. Since these options are often ineffective in the treatment of SAC, TAE might be considered as an alternative therapy to treat pain and functional limitation, before considering more aggressive interventions, although further research is warranted to elaborate robust recommendations.
Most of the studies included reported that TAE was done by interventional radiologists with more than five years of experience in embolization procedures. This remarks a certain level of dexterity required to perform the technique, which may compromise its access due to steep learning curves and unavailability of experienced interventional radiologists in all medical centers and settings.
Earlier this year, a recent systematic review on TAE treatment in frozen shoulder was published. This analysis by Digge et al.24 is limited to three studies only, two of them belonging to the same authors. Consequently, our broader review, with a larger sample size, genuinely explores several aspects of TAE for SAC not mentioned in the previous review.
This systematic review was limited by the quality of the studies included, as none of the studies are controlled randomized studies, and the sample size within each study was small. The heterogeneity in population, outcome measures, materials used, embolization technique and follow-up time did not make it possible to elaborate profound statistical analysis, including meta-analysis. SAC has been described as a self-limiting chronic condition, meaning that patients symptoms would improve without treatment with the passing of time (18-30 months), making it difficult to assesses the efficacy of TAE without considering the natural course of the disease. Randomized trials are required to evaluate the efficacy solely attributable to TAE, and also to evaluate any potential placebo effect. Furthermore, it has been shown that residual pain can last approximately 15 years36. A longer follow-up period would be needed to evaluate the long-term benefits and durability of the observed effects of TAE.
Conclusion
In conclusion, this systematic review included studies with promising results that suggest that TAE is a safe and effective treatment option for managing pain and restoring shoulder mobility in SAC. Given the lack of consensus in the management of SAC, TAE might be an innovative minimally invasive therapeutic option, especially for patients who have not improved after conventional care. However, future prospective randomized trials are required to obtain robust conclusions about the therapeutic effects of this technique, and issue evidence-based recommendations about clinical use in the management of SAC.
Authors Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Amina Ait and Sergio Barroso. The first draft of the manuscript was written by Amina Ait and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Funding
No funding sources have contributed to the completion of this article.
Competing Interests
All authors declare no conflicts of interests applicable to this report.
References
- Ricci M. Adhesive capsulitis: A review for clinicians. JAAPA. 2021; 34(12): 12-14. doi:10.1097/01.JAA.0000800236.81700.D4
- D’Orsi GM, Giai Via A, Frizziero A, et al. Treatment of adhesive capsulitis: a review. Muscles Ligaments Tendons J. 2012; 2(2): 70. Accessed August 5, 2022. /pmc/articles/PMC3666515/
- Ewald A. Adhesive Capsulitis: A Review. Am Fam Physician. 2011; 83(4): 417-422. Accessed August 5, 2022. www.aafp.org/afp
- Jump CM, Duke K, Malik RA, et al. Frozen Shoulder. JBJS Rev. 2021; 9(1): e19.00153. doi:10.2106/JBJS.RVW.19.00153
- Sasanuma H, Sugimoto H, Iijima Y, et al. Blood flow evaluation by dynamic magnetic resonance imaging of symptomatic rotator cuff tears and frozen shoulders. J Shoulder Elbow Surg. 2018; 27(12): e372-e379. doi:10.1016/J.JSE.2018.05.042
- Sasanuma H, Sugimoto H, Fujita A, et al. Characteristics of dynamic magnetic resonance imaging of idiopathic severe frozen shoulder. J Shoulder Elbow Surg. 2017; 26(2): e52-e57. doi:10.1016/J.JSE.2016.06.003
- Ramirez J. Adhesive Capsulitis: Diagnosis and Management. Am Fam Physician. 2019; 99(5): 297-300.
- Vastamäki H, Kettunen J, Vastamäki M. The natural history of idiopathic frozen shoulder: a 2- to 27-year followup study. Clin Orthop Relat Res. 2012; 470(4): 1133-1143. doi:10.1007/S11999-011-2176-4
- Grey R. The natural history of “idiopathic” frozen shoulder. J Bone Joint Surg Am. 1978; 60(4): 564.
- Higgins J, Thomas J, Chandler J, et al. Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (Updated February 2021). Cochrane; 2021.
- Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021; 372: n71. doi:10.1136/BMJ.N71
- Sterne JA, Hernán MA, Reeves BC, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016; 355: i4919. doi:10.1136/BMJ.I4919
- McGuinness LA, Higgins JPT. Risk-of-bias VISualization (robvis): An R package and Shiny web app for visualizing risk-of-bias assessments. Res Synth Methods. 2021; 12(1): 55-61. doi:10.1002/JRSM.1411
- Chapman CR, Casey KL, Dubner R, et al. Pain measurement: an overview. Pain. 1985; 22(1): 1-31. doi:10.1016/0304-3959(85)90145-9
- Bagla S, Nagda S, Piechowiak R, et al. Results from a United States Investigational Device Study of Adhesive Capsulitis Embolization in the Treatment of Shoulder Pain: The Adhesive Capsulitis Embolization Study. J Vasc Interv Radiol. 2022; 33(2): 177-182. doi:10.1016/J.JVIR.2021.10.031
- Okuno Y, Iwamoto W, Matsumura N, et al. Clinical Outcomes of Transcatheter Arterial Embolization for Adhesive Capsulitis Resistant to Conservative Treatment. J Vasc Interv Radiol. 2017; 28(2): 161-167.e1. doi:10.1016/J.JVIR.2016.09.028
- Fernández Martínez AM, Baldi S, Alonso-Burgos A, et al. Mid-Term Results of Transcatheter Arterial Embolization for Adhesive Capsulitis Resistant to Conservative Treatment. Cardiovasc Intervent Radiol. 2021; 44(3): 443-451. doi:10.1007/S00270-020-02682-4
- Ciampi-Dopazo J, Puentes-Gutierrez A, Marquina-Valero M, et al. Combined transcatheter arterial embolization and rehabilitative treatment for adhesive capsulitis refractory to conservative treatment. Intervencionismo. 2020; 20(2): 74-83. doi:10.30454/2530-1209.2020.2.2
- Fernández-Martínez AM, Alonso-Burgos A, López R, et al. Clinical Outcomes of Transcatheter Arterial Embolization for Secondary Stiff Shoulder. J Vasc Interv Radiol. 2021; 32(4): 489-496. doi:10.1016/J.JVIR.2020.11.006
- Okuno Y, Oguro S, Iwamoto W, et al. Short-term results of transcatheter arterial embolization for abnormal neovessels in patients with adhesive capsulitis: a pilot study. J Shoulder Elbow Surg. 2014; 23(9): e199-206. doi:10.1016/J.JSE.2013.12.014
- Sajan A, Isaacson A, Bagla S. Adhesive Capsulitis Embolization: A Case of Particle Embolization for Refractory Shoulder Pain Secondary to Adhesive Capsulitis. J Radiol Nurs. 2021; 40(2): 136-138. doi:10.1016/J.JRADNU.2021.01.003
- Gremen E, Frandon J, Lateur G, et al. Safety and Efficacy of Embolization with Microspheres in Chronic Refractory Inflammatory Shoulder Pain: A Pilot Monocentric Study on 15 Patients. Biomedicines. 2022; 10(4): 744. doi:10.3390/BIOMEDICINES10040744
- Okuno Y, Matsumura N, Oguro S. Transcatheter arterial embolization using imipenem/cilastatin sodium for tendinopathy and enthesopathy refractory to nonsurgical management. J Vasc Interv Radiol. 2013; 24(6): 787-792. doi:10.1016/J.JVIR.2013.02.033
- Digge V, Kumar V, Kar S, et al. Is there evidence to recommend transcatheter arterial embolisation in adhesive capsulitis: A review of literature. J Orthop. 2022; 30: 77-82. doi:10.1016/J.JOR.2022.02.008
- Sajan A, Bagla S, Isaacson A. Non-neoplastic Disease Outside the Spine-Genicular Artery Embolization and Adhesive Capsulitis Embolization. Tech Vasc Interv Radiol. 2020; 23(4): 100702. doi:10.1016/J.TVIR.2020.100702
- Okuno Y, Iwamoto W, Matsumura N, et al. Mid-term outcomes of prospective clinical trial of transcatheter arterial micro embolization (TAME) for resistant frozen shoulder. J Shoulder Elbow Surg. 2017; 26(4): e113. doi:10.1016/J.JSE.2016.11.031
- Lee SH, Hwang JH, Kim DH, et al. Clinical Outcomes of Transcatheter Arterial Embolisation for Chronic Knee Pain: Mild-to-Moderate Versus Severe Knee Osteoarthritis. Cardiovasc Intervent Radiol. 2019; 42(11): 1530-1536. doi:10.1007/S00270-019-02289-4
- Okuno Y, Korchi AM, Shinjo T, et al. Transcatheter arterial embolization as a treatment for medial knee pain in patients with mild to moderate osteoarthritis. Cardiovasc Intervent Radiol. 2015; 38(2): 336-343. doi:10.1007/S00270-014-0944-8
- Hindsø L, Riis RGC, Hölmich P, et al. Current Status of Trans-Arterial Embolization in Pain Management of Musculoskeletal Inflammatory Conditions - An Evidence-Based Review. Cardiovasc Intervent Radiol. 2021; 44(11): 1699-1708. doi:10.1007/S00270-021-02948-5
- Costa C, Incio J, Soares R. Angiogenesis and chronic inflammation: Cause or consequence? Angiogenesis. 2007; 10(3): 149-166. doi:10.1007/S10456-007-9074-0
- Alfredson H, Öhberg L, Forsgren S. Is vasculo-neural ingrowth the cause of pain in chronic Achilles tendinosis? An investigation using ultrasonography and colour Doppler, immunohistochemistry, and diagnostic injections. Knee Surg Sports Traumatol Arthrosc. 2003; 11(5): 334-338. doi:10.1007/S00167-003-0391-6
- Mapp PI, Walsh DA. Mechanisms and targets of angiogenesis and nerve growth in osteoarthritis. Nat Rev Rheumatol. 2012; 8(7): 390-398. doi:10.1038/NRRHEUM.2012.80
- Chellathurai A, Subbiah K, Elangovan A, et al. Adhesive capsulitis: MRI correlation with clinical stages and proposal of MRI staging. Indian J Radiol Imaging. 2019; 29(1): 19-24. doi:10.4103/IJRI.IJRI_116_18
- Pessis E, Mihoubi F, Feydy A, et al. Usefulness of intravenous contrast-enhanced MRI for diagnosis of adhesive capsulitis. Eur Radiol. 2020; 30(11): 5981-5991. doi:10.1007/S00330-020-07003-4
- Filippiadis DK, Binkert C, Pellerin O, et al. Cirse Quality Assurance Document and Standards for Classification of Complications: The Cirse Classification System. Cardiovasc Intervent Radiol. 2017; 40(8): 1141-1146. doi:10.1007/S00270-017-1703-4
- Farrell CM, Sperling JW, Cofield RH. Manipulation for frozen shoulder: long-term results. J Shoulder Elbow Surg. 2005; 14(5): 480-484. doi:10.1016/J.JSE.2005.02.012
