Cold shock RNA-binding protein RBM3 as a potential therapeutic target to prevent skeletal muscle atrophy
Douglas W. Van Pelt, Zachary R. Hettinger, Esther E. Dupont-Versteegden*
Department of Physical Therapy and Center for Muscle Biology, University of Kentucky, Lexington, KY 40536, USA
Abstract
Muscle atrophy is among the most common conditions during sickness, injury, aging and after orthopedic surgeries, and is associated with poor health outcomes. As such, it is important to understand the molecular machinery responsible for the control of muscle mass and function for development of therapeutic targets and strategies to combat muscle atrophy. We have identified the cold shock RNA binding protein, RNA-binding motif protein 3 (RBM3) as a critical regulatory node in the control of skeletal muscle mass and herein, we review our current knowledge of its actions in skeletal muscle. We also cover future directions of research and how this knowledge may translate into therapeutic interventions.
The pleotropic nature of RNA-binding proteins and RBM3
Maintaining skeletal muscle mass throughout the lifespan is important for overall metabolic health1 and for preserving health span with aging2,3. In addition, muscle atrophy after orthopedic surgery contributes to sustained weakness that can impede recovery4. Our laboratory has identified the cold shock RNA binding protein (RBP), RNA-binding motif protein 3 (RBM3) as a regulatory node in the control of skeletal muscle mass5,6, suggesting it may be a promising target for therapies combating muscle atrophy. RBM3 is a RBP that has been shown to have multiple functions. As a whole, RBPs play a role in governing RNA metabolism and post-transcriptional processes related to mRNA polyadenylation, alternative splicing, nucleocytoplasmic trafficking, stability, and translation initiation and elongation7-10, which ultimately affects protein synthesis11. It is therefore not surprising that RBM3 is involved in various cell types, with a variety of functions that are mostly protective or beneficial which include, enhancing translational efficiency and protein synthesis8,12,13, miRNA biogenesis14, cell migration15, response to hypoxia16, and protection against necrosis and apoptosis17-19.
Of particular interest with regard to skeletal muscle atrophy is understanding how RBPs augment cellular responses to stress via their impact on RNA stability. The stability of mRNAs determines not only their half-life, but the capacity of RNAs to be translated11. This feature of RBPs allows for transcript-specific translational reprogramming to create tailored and specific responses to a particular cellular stressor20. As an example, RBPs selectively protect mRNAs related to cell survival to preserve their translation in response to a DNA damage event that causes global reductions in protein synthesis21. Similar preservation of select RNAs via RBPs occurs in response to hypoxic conditions and allows for the RNAs complexed with RBPs to evade the impact of RNA degradation and reduced translation caused by hypoxia22. As such, the RBP cytosolic poly(A)-binding protein 1 (PABPC1) elevates protein synthesis and causes hypertrophy in cardiac muscle by increasing RNA stability and translational efficiency in a transcript-specific manner23,24. Although the number of studies focused on the role of RBPs in skeletal muscle atrophy are limited, we propose that dynamic changes in RBPs (such as RBM3) play a central role in muscle adaptive processes through their differential effects on mRNA stability25.
Hibernating animals demonstrate RBP-mediated prevention of muscle atrophy
The function of RBM3 as a RNA stabilizing agent is cold-inducible, hence referring to the RNA-binding protein as a “cold-shock” protein. RBM3 expression is elevated in response to cellular and body temperature dropping below 37oC and can provide a transcript-specific preservation of RNA important for cell survival and metabolic function during periods of cold stress17,26-28. Thus, it is not surprising that RBM3 is consistently upregulated in skeletal muscle and other organs of hibernating animals undergoing large drops in body temperature (sometimes reaching as low as 0-5oC)26,29-32. During hibernation, there are vast global reductions in metabolism and transcription in response to the cold temperatures and lack of physical activity, however, hibernating animals can preserve the expression and translation of select mRNAs33,34. Moreover, muscle mass does not decrease as expected in response to the lack of physical activity, reduced protein synthesis, and very low caloric intake35-37. Interestingly, it has been shown that black bears can surprisingly retain protein balance and protein content throughout the hibernation process29. Translational reprogramming via mRNA-RBP interactions (such as those seen with RBM3) is postulated to be an important factor allowing for the preservation of muscle mass25. That is, RBPs such as RBM3 may preserve select RNA transcripts promoting muscle hypertrophy in the face of the catabolic state caused by the global reductions in metabolism that occur with hibernation.
Overexpression of RBM3 promotes hypertrophy and prevents atrophy
In a study from 2008, our laboratory discovered that skeletal muscle RBM3 expression was between 2.5 to 4-fold higher in response to disuse atrophy6. The higher levels of RBM3 in atrophied muscle were apparent in both young and aged rats that underwent hindlimb suspension suggesting that the response of RBM3 is ubiquitous across age. It was postulated that the increased expression was a compensatory response in an attempt to combat atrophy since the elevation in RBM3 occurred after the appearance of apoptotic nuclei and elevations in markers of protein degradation.
These findings prompted a study to investigate whether overexpression of RBM3 prior to an atrophy event could attenuate the degree of muscle loss5. This concept is clinically important since pretreatment to attenuate atrophy would eliminate the need for muscle enhancing treatment after a period of disuse. Overexpression of RBM3 in C2C12 myotubes, an in vitro model of muscle, attenuated dexamethasone-induced atrophy. Additionally, in vivo overexpression of RBM3 in the hindlimb musculature of rats reduced the amount of muscle atrophy in response to 14 days of hindlimb suspension. The in vitro and in vivo experiments support the idea that RBM3 is indeed a protective factor against muscle loss. Interestingly, we also observed that RBM3 did not just attenuate atrophy, but promoted hypertrophy. C2C12 myotubes that overexpressed RBM3 but were not exposed to dexamethasone were 1.6-fold larger than myotubes not overexpressing RBM3. Similarly, myofibers in rat muscle under normal weight-bearing conditions that overexpressed RBM3 experienced growth. The findings from this study were the first to demonstrate that RBM3 has a direct role in skeletal muscle hypertrophy and atrophy.
Possible mechanisms dictating the anti-atrophic and hypertrophic effects of RBM3
As mentioned above, RBM3 has been demonstrated to have an abundance of functions across many cell types. One of the potential ways by which RBM3 may promote hypertrophy and prevent atrophy is by impacting protein turnover, that is, increasing protein synthesis or reducing protein degradation. RBM3 may act as a protective chaperone of mRNA by preventing their degradation and facilitating their translation to promote hypertrophy25,28,38. The tuning of protein turnover can also be independent of RNA stability as it has been shown that RBM3 enhances protein synthesis by increasing polysome formation and activation of translation initiation factors8,12. In addition, RBM3 influences the maturation and overall expression of miRNAs that are important for regulation of skeletal muscle protein degradation and ultimately muscle size14. Specifically, overexpression of RBM3 has been shown to elevate expression of miR-23a and miR-27a. MiR-23a suppresses the expression of Muscle Atrophy F-box (MAFbx, or atrogin-1) and Muscle RING finger-1 (MuRF1), two ubiquitin ligases involved in the degradation of skeletal muscle protein during atrophy39,40. A validated target of miR-27a is myostatin, a well-known negative regulator of muscle size41,42. Promoting lower protein degradation may also be initiated by RBM3’s parallel influence on reducing caspase expression and activity17, p38MAPK signaling43, and the unfolded protein response8,44. The multitude of mechanisms by which RBM3 can influence protein turnover and mRNA translation make it highly likely that it is an important modulator of the response to atrophic and hypertrophic conditions in muscle. A schematic summarizing the possible mechanisms by which RBM3 may alter protein balance and attenuate muscle atrophy is summarized in Figure 1.
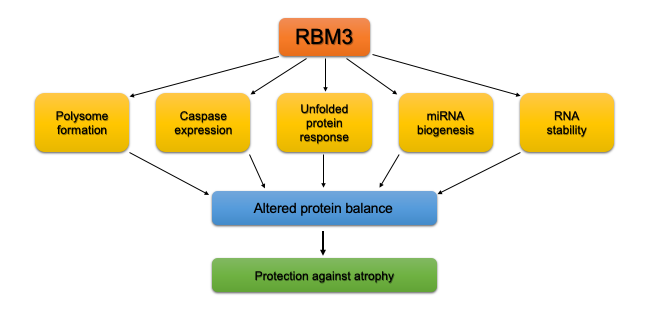
Figure 1: Mechanisms by which RBM3 may attenuate atrophy in skeletal muscle.
The cumulative impact of RBM3 on polysome formation, caspase expression and activity, unfolded protein response, miRNA biogenesis, and RNA stability can lead to a change in protein balance that ultimately protects against muscle atrophy.
Therapeutic potential of RBM3
The ultimate goal of understanding RBM3 and its role in regulating skeletal muscle mass is to derive a viable therapeutic intervention that leverages the cold shock RNA-binding protein to preserve or increase muscle mass. The ubiquity of RBPs and their involvement with a myriad of disease and pathologic conditions has made RBPs a desirable target for future pharmaceutical and gene therapy interventions across a range of disorders45. There can be a large benefit of targeting RBM3 for prevention of muscle atrophy in patients in the ICU or that are recovering from surgery. In addition, there may be therapeutic potential for RBM3 in patients with atrophy due to neuromuscular disease (e.g. ALS or muscular dystrophy) because RBM3 is also inhibitory to apoptosis in muscle17. However, mainstream usage of these types of interventions will take much more time before they can be safely and routinely implemented as a standard of care. An intriguing aspect of RBM3 is its response to cold stress and we posit that utilizing this knowledge may be the most advantageous and safe manner to leverage the potential therapeutic benefits of RBM3 in muscle wasting and muscle growth in the near future.
There is great interest in utilizing cold-water immersion to facilitate the expedited recovery of skeletal muscle following strenuous exercise bouts. The combined knowledge that cold stress can upregulate expression of RBM326,29-32 and that overexpression of RBM3 causes hypertrophy5 leads to speculation that post-exercise cold treatment may promote further gains in muscle growth and adaptation to exercise. However, current evidence regarding cold treatment following exercise remains equivocal and tends to skew towards a negative effect of cold treatment on skeletal muscle adaptations to resistance exercise (i.e. strength and mass) (reviewed)46. Surprisingly, instead of enhancing mRNA translation, the post-exercise cold treatment appears to “freeze-down” mRNA translational efficiency and capacity (as coined by Figueiredo and von Walden)47 by impeding ribosome biogenesis48,49 and subsequently lowering rates of protein synthesis48,50. Collectively, these findings promote that usage of cold stress (with the assumption that it elevates RBM3) may not be a beneficial strategy to augment muscle skeletal muscle hypertrophy in response to exercise training.
We caution the interpretation that utilizing cold stress to elevate RBM3 and promote preservation of muscle mass during atrophy causing scenarios is futile however, as there are many limitations to the aforementioned studies. First, the context of these studies is specific to application of cold stress after exercise bouts in relatively healthy and young individuals. There are no studies to date that have examined how the application of cold can mitigate atrophy during disuse or pathological conditions. Additionally, the response to cold treatment may differ among other populations (i.e. aged, ICU patients, etc.). Also, some of the studies showing that cold application lowers rates of protein synthesis apply the cold for very long periods of time that are not practical or clinically relevant (i.e. 24-48 hours of cold exposure)46,48. Lastly, no data are available to show whether cold immersion had any impact on RBM3 levels in skeletal muscle in these studies and additionally, the protocols applied may not have been sufficient to alter RBM3. Therefore, studies specifically examining the impact of clinically relevant cold application strategies should be performed to ascertain whether cold can attenuate atrophy. Even if cold-treatment in non-hibernating animals is not sufficient to elevate RBM3 and improve muscle hypertrophy in response to overload, the abundance of positive effects demonstrated by RBM3 in muscle and other cell types suggest that a mimetic or pharmaceutical intervention targeting RBM3 could be hugely beneficial for skeletal muscle.
Conclusion
The accumulation of knowledge on functions of RBM3 in skeletal muscle has highlighted its impact on muscle mass regulation. As a cold shock protein and RNA binding protein, RBM3 has the ability to influence numerous aspects of protein synthesis, through binding components necessary for protein synthesis and also by preventing RNA degradation. Through these functions, it is highly likely that RBM3 and by extension cold stress could be used as a therapeutic strategy to combat skeletal muscle atrophy. Future studies should continue to determine the specific role of RBM3 in regulation muscle mass and how this can be leveraged in generating therapies for skeletal muscle.
References
- Kalyani RR, Corriere M, Ferrucci L. Age-related and disease-related muscle loss: the effect of diabetes, obesity, and other diseases. The Lancet Diabetes & Endocrinology. 2014; 2(10): 819-29.
- Rantanen T, Guralnik JM, Sakari-Rantala R, et al. Disability, physical activity, and muscle strength in older women: the Women's Health and Aging Study. Arch Phys Med Rehabil. 1999; 80(2): 130-5.
- Rantanen T, Harris T, Leveille SG, et al. Muscle strength and body mass index as long-term predictors of mortality in initially healthy men. J Gerontol A Biol Sci Med Sci. 2000; 55(3): M168-73.
- Thomas AC, Wojtys EM, Brandon C, et al. Muscle atrophy contributes to quadriceps weakness after anterior cruciate ligament reconstruction. J Sci Med Sport. 2016; 19(1): 7-11.
- Van Pelt DW, Confides AL, Judge AR, et al. Cold shock protein RBM3 attenuates atrophy and induces hypertrophy in skeletal muscle. J Muscle Res Cell Motil. 2018; 39(1-2): 35-40.
- Dupont-Versteegden EE, Nagarajan R, Beggs ML, et al. Identification of cold-shock protein RBM3 as a possible regulator of skeletal muscle size through expression profiling. Am J Physiol Regul Integr Comp Physiol. 2008; 295(4): R1263-73.
- Harvey R, Dezi V, Pizzinga M, et al. Post-transcriptional control of gene expression following stress: the role of RNA-binding proteins. Biochem Soc Trans. 2017; 45(4): 1007-14.
- Smart F, Aschrafi A, Atkins A, et al. Two isoforms of the cold-inducible mRNA-binding protein RBM3 localize to dendrites and promote translation. J Neurochem. 2007; 101(5): 1367-79.
- Mangus DA, Evans MC, Jacobson A. Poly(A)-binding proteins: multifunctional scaffolds for the post-transcriptional control of gene expression. Genome Biol. 2003; 4(7): 223.
- Cassola A, Noe G, Frasch AC. RNA recognition motifs involved in nuclear import of RNA-binding proteins. RNA Biol. 2010; 7(3): 339-44.
- Roy B, Jacobson A. The intimate relationships of mRNA decay and translation. Trends Genet. 2013; 29(12): 691-9.
- Dresios JA, Owens A, Vanderklish GC, et al. Cold stress-induced protein Rbm3 binds 60S ribosomal subunits, alters microRNA levels, and enhances global protein synthesis. PNAS. 2005; 102(6): 5.
- Cok SJ, Acton SJ, Sexton AE, et al. Identification of RNA-binding proteins in RAW 264.7 cells that recognize a lipopolysaccharide-responsive element in the 3-untranslated region of the murine cyclooxygenase-2 mRNA. J Biol Chem. 2004; 279(9): 8196-205.
- Pilotte J, Dupont-Versteegden EE, Vanderklish PW. Widespread regulation of miRNA biogenesis at the Dicer step by the cold-inducible RNA-binding protein, RBM3. PLoS One. 2011; 6(12): e28446.
- Pilotte J, Kiosses W, Chan SW, et al. Morphoregulatory functions of the RNA-binding motif protein 3 in cell spreading, polarity and migration. Sci Rep. 2018; 8(1): 7367.
- Wellmann S, Buhrer C, Moderegger E, et al. Oxygen-regulated expression of the RNA-binding proteins RBM3 and CIRP by a HIF-1-independent mechanism. J Cell Sci. 2004; 117(Pt 9): 1785-94.
- Ferry AL, Vanderklish PW, Dupont-Versteegden EE. Enhanced survival of skeletal muscle myoblasts in response to overexpression of cold shock protein RBM3. Am J Physiol Cell Physiol. 2011; 301(2): C392-402.
- Pilotte J, Cunningham BA, Edelman GM, et al. Developmentally regulated expression of the cold-inducible RNA-binding motif protein 3 in euthermic rat brain. Brain Res. 2009; 1258: 12-24.
- Peretti D, Bastide A, Radford H, et al. RBM3 mediates structural plasticity and protective effects of cooling in neurodegeneration. Nature. 2015; 518(7538): 236-9.
- Liu B, Qian SB. Translational reprogramming in cellular stress response. Wiley Interdiscip Rev RNA. 2014; 5(3): 301-15.
- Dutertre M, Lambert S, Carreira A, et al. DNA damage: RNA-binding proteins protect from near and far. Trends Biochem Sci. 2014; 39(3): 141-9.
- Uniacke J, Holterman CE, Lachance G, et al. An oxygen-regulated switch in the protein synthesis machinery. Nature. 2012; 486(7401): 126-9.
- Chorghade S, Seimetz J, Emmons R, et al. Poly(A) tail length regulates PABPC1 expression to tune translation in the heart. Elife. 2017; 6.
- Smith RW, Blee TK, Gray NK. Poly(A)-binding proteins are required for diverse biological processes in metazoans. Biochem Soc Trans. 2014; 42(4): 1229-37.
- Van Pelt DW, Hettinger ZR, Vanderklish PW. RNA-binding proteins: The next step in translating skeletal muscle adaptations? J Appl Physiol (1985). 2019; 127(2): 654-60.
- Danno S, Nishiyama H, Higashitsuji H, et al. Increased transcript level of RBM3, a member of the glycine-rich RNA-binding protein family, in human cells in response to cold stress. Biochem Biophys Res Commun. 1997; 236(3): 804-7.
- Phadtare S, Alsina J, Inouye M. Cold-shock response and cold-shock proteins. Curr Opin Microbiol. 1999; 2(2): 175-80.
- Al-Fageeh MB, Smales CM. Control and regulation of the cellular responses to cold shock: the responses in yeast and mammalian systems. Biochem J. 2006; 397(2): 247-59.
- Fedorov VB, Goropashnaya AV, Toien O, et al. Elevated expression of protein biosynthesis genes in liver and muscle of hibernating black bears (Ursus americanus). Physiol Genomics. 2009; 37(2): 108-18.
- Vadim B Fedorov AVG, Øivind Tøien, Nathan C Stewart, et al. Modulation of gene expression in heart and liver of hibernating black bears (Ursus americanus). BMC Genomics. 2011; 12(171).
- Williams DR, Epperson LE, Li W, et al. Seasonally hibernating phenotype assessed through transcript screening. Physiol Genomics. 2005; 24(1): 13-22.
- Yan J, Barnes BM, Kohl F, et al. Modulation of gene expression in hibernating arctic ground squirrels. Physiol Genomics. 2008; 32(2): 170-81.
- Tessier SN, Storey KB. To be or not to be: the regulation of mRNA fate as a survival strategy during mammalian hibernation. Cell Stress Chaperones. 2014; 19(6): 763-76.
- Hittel D, Storey KB. The translation state of differentially expressed mRNAs in the hibernating 13-lined ground squirrel (Spermophilus tridecemlineatus). Arch Biochem Biophys. 2002; 401(2): 244-54.
- Lohuis TD HH, Beck TDI, Iaizzo PA. Hibernating Bears Conserve Muscle Strength and Maintain Fatigue Resistance. Physiological and Biochemical Zoology: Ecological and Evolutionary Approaches. 2007; 80(3): 12.
- Lee K, Park JY, Yoo W, et al. Overcoming muscle atrophy in a hibernating mammal despite prolonged disuse in dormancy: proteomic and molecular assessment. J Cell Biochem. 2008; 104(2): 642-56.
- Rourke BC YY, Milsom WK, Caiozzo VJ. Myosin Isoform Expression and MAFbx mRNA Levels in Hibernating GoldenâMantled Ground Squirrels (Spermophilus lateralis). Physiological and Biochemical Zoology: Ecological and Evolutionary Approaches. 2004; 77(4): 11.
- Gualerzi CO, Maria Giuliodori A, Pon CL. Transcriptional and Post-transcriptional Control of Cold-shock Genes. Journal of Molecular Biology. 2003; 331(3): 527-39.
- Wada S, Kato Y, Okutsu M, et al. Translational suppression of atrophic regulators by microRNA-23a integrates resistance to skeletal muscle atrophy. J Biol Chem. 2011; 286(44): 38456-65.
- Hudson MB, Woodworth-Hobbs ME, Zheng B, et al. miR-23a is decreased during muscle atrophy by a mechanism that includes calcineurin signaling and exosome-mediated export. Am J Physiol Cell Physiol. 2014; 306(6): C551-8.
- Huang Z, Chen X, Yu B, et al. MicroRNA-27a promotes myoblast proliferation by targeting myostatin. Biochem Biophys Res Commun. 2012; 423(2): 265-9.
- Allen DL, Loh AS. Posttranscriptional mechanisms involving microRNA-27a and b contribute to fast-specific and glucocorticoid-mediated myostatin expression in skeletal muscle. Am J Physiol Cell Physiol. 2011; 300(1): C124-37.
- Yang HJ, Ju F, Guo XX, et al. RNA-binding protein RBM3 prevents NO-induced apoptosis in human neuroblastoma cells by modulating p38 signaling and miR-143. Sci Rep. 2017; 7: 41738.
- Zhu X, Zelmer A, Kapfhammer JP, et al. Cold-inducible RBM3 inhibits PERK phosphorylation through cooperation with NF90 to protect cells from endoplasmic reticulum stress. FASEB J. 2016; 30(2): 624-34.
- Nussbacher JK, Tabet R, Yeo GW, et al. Disruption of RNA Metabolism in Neurological Diseases and Emerging Therapeutic Interventions. Neuron. 2019; 102(2): 294-320.
- Broatch JR, Petersen A, Bishop DJ. The Influence of Post-Exercise Cold-Water Immersion on Adaptive Responses to Exercise: A Review of the Literature. Sports Med. 2018; 48(6): 1369-87.
- Figueiredo VC, von Walden F. Freezing translation at multiple levels one session at a time. J Physiol. 2020; 598(8): 1441-2.
- Rantala R, Chaillou T. Mild hypothermia affects the morphology and impairs glutamine-induced anabolic response in human primary myotubes. Am J Physiol Cell Physiol. 2019; 317(1): C101-C10.
- Figueiredo VC, Roberts LA, Markworth JF, et al. Impact of resistance exercise on ribosome biogenesis is acutely regulated by post-exercise recovery strategies. Physiol Rep. 2016; 4(2).
- Fuchs CJ, Kouw IWK, Churchward-Venne TA, et al. Postexercise cooling impairs muscle protein synthesis rates in recreational athletes. J Physiol. 2020; 598(4): 755-72.
